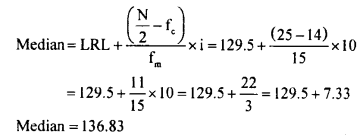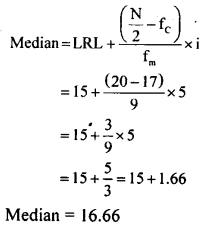Students can Download KSEEB Class 8 Hindi गिनतियाँ, KSEEB Solutions for Class 8 Hindi helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka State Syllabus Class 8 Hindi गिनतियाँ


इकाई – 1
दहाई – 10
सैकड़ा – 100
हज़ार – 1000
दस हज़ार – 10,000
लाख – 1,00, 000
करोड़ – 100,00,000
अरब (बिलियन) – 1,000,000,000
सौ अरब-एक नीलम – (100,000,000,000)
- पहला, दूसरा, तीसरा, चौथा, पाँचवाँ, छठा (छठ्वाँ), सातवाँ, आठवाँ, नौवाँ, दसवाँ।
- पहली, दूसरी, तीसरी, चौथी, पाँचवीं, छठी, सातवीं, आठवीं, नौवीं, दसवीं ।
- प्रथम, द्वितीय, तृतीय, चतुर्थ, पंचम, षष्ठ, सप्तम, अष्टम, नवम, दशम
- क्षण = 3 मिनट
- मन = 12 किलो
- एक सेर = 933.10 ग्रॉम = 0.937 लीटर
- छटक = 58.125 ग्राम (app. 59 gms)
- 1 मील = 1.61 कि.मी.
समयावधि
10 वर्ष – दशाब्दी (decade)
12 वर्ष – युग (period/age of 12 years)
100 वर्ष – शताब्दी (century)
पखवाड़ा/पाक्षिक – 15 दिन (fortnight)
महीना/माह/मास – 30 दिन (month)
1 वर्ष – 365 दिन (year)