Students can Download Karnataka SSLC Science Previous Year Question Paper 2019 5 with Answers, Karnataka SSLC Science Previous Year Question Paper 2019s with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka State Syllabus SSLC Science Previous Year Question Paper March 2019
Time: 3 Hours
Max Marks: 80
Question 1.
The change that occurs in the eye to see the distant objects clearly is
a. focal length of the eye lens decreases
b. curvature of the eye lens decreases
c. focal length of the eye lens increases
d. ciliary muscles of the eye contract.
Answer:
c. focal length of the eye lens increases
Question 2.
The functional groups present in propanol and propanal respectively are.
a. -OH and -CHO
b. -OH and – COOH
c. – COP and COOH
d. – CHO and – CO
Answer:
a. – OH and – CHO
Question 3.
The correct path of the movement of nerve impulses in the following diagram is
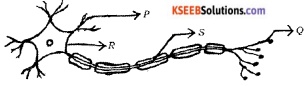
(A) Q → S → R → P
(B) P → Q → R → S
(C) S → R → Q → P
(D) P → R → S → Q
Answer:
(D) P → R → S → Q
![]()
Question 4.
The resistance of a conductor is 27. If it is cut into three equal parts and connected in parallel, then its total resistance is
a. 6 Ω
b. 3 Ω
c. 9 Ω
d 27 Ω
Answer:
b. 3 Ω
Question 5.
The chemical equation that represents neutralization reaction among the following is
a. BaCl2 + H2SO4 → BaSO4 + 2HCl
b. MnO2 + 4 HCl → MnCl2 + 2H2O + Cl2
c. 2 NaOH + H2SO4 → NaSO4 + 2H2O
d AgNO3 + HCl → AgCl + HNO3
Answer:
c. 2 NaOH + H2SO4 → NaSO4 + 2H2O
Question 6.
By constructing Khadin check-dams in level terrains,
a. underground water level decreases
b. underground water level increases
c. vegetation in the nearby areas are destroyed due to excess moisture
d. underground water gets polluted
Answer:
b. underground water level increases
Question 7.
To obtain a diminished image of an object from a concave mirror, position of the object should be
(F = principal focus, C = centre of curvature, P – pole)
a. between C and F
b. beyond C
c. between P and F
d. at F
Answer:
b. beyond C
Question 8.
The electronic configuration of element x is 2, 8, 8, 1 and the electronic configuration of element Y is 2, 8, 7. Then the type of bond formed between these two elements is
a. covalent bond
b. hydrogen bond
c. metallic bond
d. ionic bond
Answer:
d. ionic bond
Question 9.
Part of the flower that develops into fruit and part of the seed that develops into root respectively are
(A) ovary and plumule
(B) plumule and radicle
(C) ovary and radicle
(D) ovary and ovule
Answer:
(C) ovary and radicle.
Question 10.
A pure dominant pea plant producing round – yellow seeds is crossed with pure recessive pea plant producing wrinkled – green seeds. The number of plants bearing round – green seeds in the F1 generation of Mendel’s experiment is
(A) 0
(B) 1
(C) 3
(D) 9
Answer:
(A) 0
![]()
Question 11.
The functions of hormones are given in Column-A and the names of. the hormones are given in Column-B. Match them and write the answer along with its letters :
| Column – A | Column – B |
| (A) Prepares the body to deal with the situation | (i) Growth hormone |
| (B) Regulates metabolism for body growth | (ii) Testosterone |
| (C) Regulates blood sugar levels | (iii) Adrenaline |
| (D) Regulates the growth and development of the body | (iv) Progesterone |
| (v) Insulin | |
| (vi) Thyroxine | |
| (vii) Oestrogen |
Answer:
(A) – (iii) Adrenaline
(B) – (vi) Thyroxine
(C) – (v) Insulin
(D) – (i) Growth hormone
Question 12.
Name the acid present in the stinging hairs of nettle leaves.
Answer:
Methanoic acid.
Question 13.
What are fossils ?
Answer:
The preserved traces of the living organisms are called fossils.
Question 14.
Convex mirror is commonly used as rear – view mirror in vehicles. Why?
Answer:
- They always give an erect diminished image.
- Also they have a wider field of view as they are curved outwards.
Question 15.
What is roasting in metallurgy?
Answer:
The sulphide ores are converted into oxides by heating strongly in the presence of excess air.
Question 16.
Observe the given figure. Name the eye defect indicated in the figure and also mention the lens used to correct this defect.
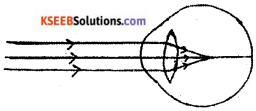
Answer:
- Myopia
- Concave lens
Question 17.
What is Tyndall effect?
Answer:
The phenomenon of scattering of light by the colloidal particles gives rise to tyndall effect.
![]()
Question 18.
Under what condition lactic acid is produced in the muscle cells ?
Answer:
Lactic acid is produced when there is lack of oxygen in the muscle cells.
Question 19.
Draw the diagram of an electric circuit in which the resistors R1, R2 and R3 are connected in parallel including an ammeter and a voltmeter and mark the direction of the current.
Answer:
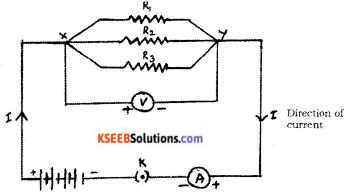
Question 20.
Name the brown fumes liberated when lead nitrate is heated. Write the balanced chemical equation for this reaction.
Answer:
Nitrogen dioxide (NO2)
2 Pb(NO3)2 → 2PbO + 4NO2 + O2
Question 21.
Explain the process of trans location of food materials in plants;
Answer:
- The transport of soluble products of photosynthesis is called trans location.
- It occurs in the phloem.
- The products of photosynthesis are transported from leaves to all parts of the plant body.
- Translocation takes place in the sieve tubes with the help of companion cells.
OR
Explain the process of digestion in the small intestine of man.
Answer:
Digestion of food in small intestine :
- Small intestine is the site of complete digestion of proteins, carbohydrates and fats.
- Glands -present in the walls of small intestine secrete intestinal juice.
- Enzymes in the intestinal juice convert proteins into amino acids, complex carbohydrates into glucose and fats into fatty acids and glycerol.
- Digested food is absorbed by the villi present in the walls of intestine.
![]()
Question 22.
Draw the diagram of a simple electric motor. Label the following parts :
i) Split rings
ii) Brushes
Answer:
i) Split rings
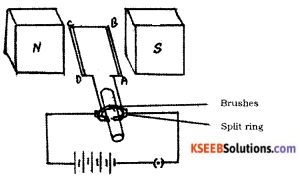
ii) Brushes
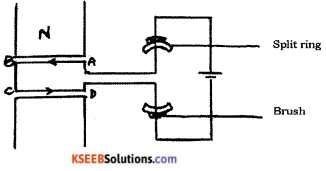
Question 23.
What are structural isomers? Name the first number of alkanes that shows structural isomerism.
Answer:
Compounds with identical molecular formula but different structures of called structural isomers.
Butane or C4H10
Question 24.
Draw the diagram showing the longitudinal section of a flower.
Label the following parts :
(i) Style (ii) Anther.
Answer:
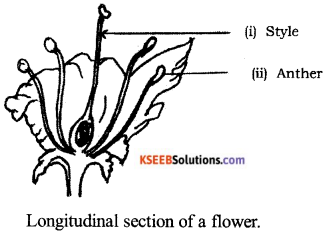
Question 25.
Draw the diagram of arrangement of apparatus used to show the reaction of zinc granules with dilute sulphuric acid and testing hydrogen gas by burning. Label the following parts.
i) Soap solution
ii) Delivery tube.
Answer:
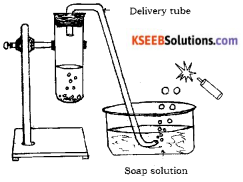
Question 26.
It is advantageous to connect electric devices in parallel instead of connecting them in series. Why?
Answer:
- The appliances connected in series need currents of widely different values to operate properly.
- In a series circuit, if one component fails, the circuit is broken and none of the components work.
- But in a parallel circuit current divides through the electrical gadgets.
- This is helpful particularly when each gadget had different resistance and requires different current to operate properly.
OR
According to Joule’s law of heating, mention the factors on which heat produced in a resistor depends. According to this law write the formula used to calculate the heat produced.
Answer:
Heat produced in a resistor is,
- Directly proportional to the square of current for a given resistance
- Directly proportional to resistance for a given current, and
- Directly proportional to the time for which the current flows through the resistor
- H – I2Rt
![]()
Question 27.
List the disadvantages of using fossil fuels.
Answer:
- Fossil fuels are formed from carbon and biomass which contains hydrogen, carbon, nitrogen and sulphur.
- When these are burnt, the products are oxides of carbon, water, oxides of nitrogen and oxides of sulphur.
- Oxides of nitrogen, oxides of sulphur and carbon monoxide are poisonous at high concentration. They may lead to acid rain.
- Carbon dioxide is a greenhouse gas. When its concentration in the atmosphere increases continuously, leads to intence global warming.
OR
List the advantages of ‘reduce’ and ‘reuse’ to save environment. Advantages of reduce and reuse to save environment:
Answer:
Reduce :By the practice of ‘Reduce’, we can save
- Electricity
- Water
- Food
- Natural resources.
Reuse : By the practice of ‘Reuse’
- Environment pollution can be controlled
- Materials are available for immediate use
- Energy can be saved
- Use of raw materials can be minimised.
Question 28.
The focal length of a concave lens is 30 cm. At what distance should the object be placed from the lens so that it forms an image at 20 cm from the lens?
Answer:
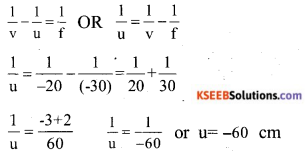
![]()
Question 29.
Draw the diagram of the apparatus used in the electrolysis of water. Label the following parts.
i) Graphite rod
ii) Cathode
Answer:
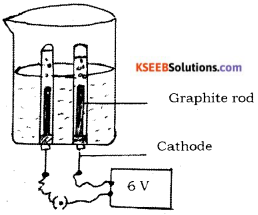
Question 30.
Growth of thread like structures along with the gradual spoilage of tomato can be observed when a cut tomato is kept aside for four days. Interpret the causes for this change.
Answer:
- The thread like structures that grow on the tomato are hyphae of Rhizopus (Bread mould)
- They have rod like structures called sporangia.
- Sporangia contain spores, they are reproductive structures.
- When spores come into contact with moist surface, they begin to grow. Therefore cut tomato gets spoiled gradually.
Question 31.
An “electric refrigerator rated 300 W is used for 8 hours a day. An electric iron box rated 750 W is used for 2 hours a day. Calculate the cost of using these appliances for 30 days, if the cost of 1 kWh is Rs. 3 /-
Answer:
The total energy consumed by the refrigerator in 30 days
= 400 × 8 × 30 = 96000 Wh = 96 kWh
The total energy consumed by the iron box in 30 days
=750 × 2 × 30 = 45000 Wh = 45 kWh
The total energy consumed by the refrigerator and iron box is
=96kWh + 45kWh = 141 kWh
The sum of bill amount for 141 kWh at rate of Rs. 3 per 1 kWh is
=141 × 3 = Rs. 423
Question 32.
There is no change in the colour of red litmus and blue litmus paper when introduced into an aqueous solution of sodium chloride. After passing direct current through the same solution, red litmus changes to blue colour. Which product is responsible for this change? Mention any two uses of this product.
Answer:
Sodium hydroxide / NaOH.
- Degreasing metals
- Soaps and detergents
- Paper making
- Artificial fibres.
![]()
Question 33.
A food chain in a polluted aquatic ecosystem is given. Observe it and answer the following question.
Fresh water → Algae → Fishes → Birds.
(i) Which organisms are disturbed more due to biomagnification ? Why ?
Answer:
- Birds are disturbed more due to biomagnification.
- As the birds occupy the top most level in the given food chain, the maximum concentration of harmful chemicals causing biomagnification get accumulated in their body.
(ii) This ecosystem will be destroyed gradually due to biomagnification. Why?
Answer:
- Biomagnification is the process of accumulation of non-degradable chemicals in the various trophic levels of food chains.
- As the chemicals are non-degradable or cannot be washed, they cannot be removed from the organisms of the food chain. This leads to gradual destruction of the ecosystem.
OR
A student places a piece of cucumber, a glass piece, a banana peel and a plastic pen in a pit and closes it. What changes can be observed in these materials after a month ? Give scientific reason for these changes.
Answer:
- Cucumber piece and banana peel are organic substances.
- They are biodegradable substances, and are eco-friendly.
- Glass piece and plastic pen are inorganic / synthetic substances.
- They are non-biodegradable substances and cause soil pollution.
Question 34.
What is dispersion of light? Mention the colour that bends the least and the colour that bends the most when light undergoes dispersion through a prism.
Answer:
The splitting of light into its component colours is called dispersion.
- The red colour bends the least.
- The voilet colour bends the most.
OR
Mention any four phenomena that can be observed due to atmospheric refraction of light on the earth.
Answer:
- The sun is visible to us two minutes before the actual sun rise.
- The sun is visible to us two minutes after the actual sunset also.
- The apparent position of the star is slightly different from its actual position.
- Twinkling of star
- Formation of rainbow
- The apparent random wavering or flickering of objects seen through a turbulent stream of hot air rising above a fire or a radiator.
![]()
Question 35.
Draw the ray diagrams for the image formation in a convex lens when an object is placed.
Answer:
i) at focus F1
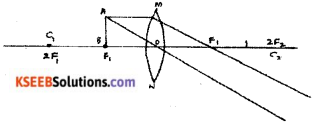
ii) beyond 2F1
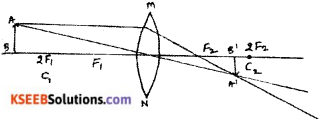
Question 36.
i. Write the differences between saturated and unsaturated hydrocarbons.
Answer:
Saturated hydrocarbons :
- In carbon compounds, carbon atoms are satisfied by a single bond between them.
- These compounds are normally not very reactive.
Unsaturated hydrocarbons :
- In carbon compounds, carbon atoms have double or triple bonds between them.
- They are more reactive than the saturated carbon compounds.
ii. Write the molecular formula and structural formula of an alkene having five carbon atoms.
Answer:
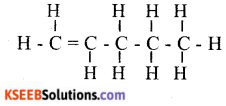
OR
i. Carbon atom does not form C4- anion and C4+ cation. Why?
Answer:
- Carbon can gain four electrons. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is four extra electrons.
- It can lose four electrons but it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
ii. How can ethanol be converted into ethanoic acid?
Answer:
Alkaline potassium permangnate or acidified potassium dichromate is added to ethyl alcohol. When it is heated it oxidises to form ethanoic acid.
![]()
![]()
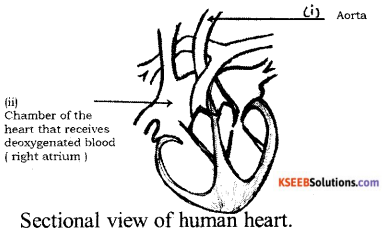
Question 37.
Draw the diagram showing the sectional view of the human heart. Label the following parts.
(i) Aorta
(ii) Chamber of the heart that receives deoxygenated blood.
Answer:
Question 38.
i. Name the major constituent of biogas. Write the properties of biogas which make it a good fuel.
Answer:
- Methane / CH4
- Leaves no residue like ash
- It bums without smoke.
- Its heating capacity is high
- Bio-gas is also used for lighting.
ii. Name the two devices that work using heat energy of the sun.
Answer:
- Solar water heater
- Solar cooker
OR
i. Write the advantages of solar cells.
Answer:
- They have no moving parts.
- Require little maintenance and work quite satisfactorily without the use of any focusing device.
- They can be set up in remote and inaccessible hamlets also.
- Very sparsely inhabited areas in which laying of a power transmission line may be expensive and not commercially viable.
ii. Write any two hazards of nuclear power generation.
Answer:
- Improper nuclear waste storage and disposal result in environmental contamination.
- There is a risk of accidental leakage of nuclear radiation.
Question 39.
Observe the given table and answer the following question :
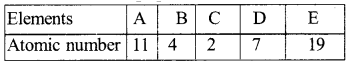
Identify the two elements that belong to the same period and the two elements that belong to the same group. Give reason for your conclusion.
Answer:
- Element B and element D are in same period because their atoms have two shells.
- Element A and element E are in the same , group because their outermost shell has one electron.
![]()
Question 40.
i) How does overload and short- circuit occur in an electric circuit? Explain. What is the function of fuse during this situation?
Answer:
- Overloading can occur when the live wire and the neutral wire come into direct contact.
- This occurs when the insulation of wires is damaged or there is a fault in the appliance.
- In such a situation, the current in the circuit abruptly increases.
- The heating that takes place in the fuse melts it to break the electric circuit and prevents the electric appliances from possible damage.
ii) Mention two properties of magnetic field lines.
Answer:
- No two field lines are found to cross each other.
- The density of the magnetic field lines are more in their poles.
- The magnetic field lines emerge from north pole and merge at south pole.
- Inside the magnet, the direction of field lines is from its south pole to its north pole.
- Thus the magnetic field lines are closed curves.
Question 41.
Give reasons :
i) Ionic compounds in solid state do not conduct electricity, whereas in molten state are good conductors of electricity.
Answer:
- In the solid state ionic compounds do not conduct electricity because movement of ions in the solid is not possible due to their rigid structure.
- In solid state, they are hard because of the strong force of attraction between the positive and negative ions.
- In molten state, electrostatic forces of attraction’ between the oppositely charged ions are overcome due to the heat.
- thus the ions move freely and conduct electricity.
ii) Silver articles when exposed to air gradually turn blackish.
Answer:
Silver reacts with sulphur in the air to form a coating of silver sulphide.
iii) Chemical reaction does not take place when copper is added to iron sulphate solution.
Answer:
Reactivity of copper is less than that of iron.
OR
Give reasons :
i) “Alloys of iron are more useful when compared to pure iron.”
Answer:
- Pure iron is very soft.
- Streches easily when hot.
- Alloys are hard.
- The properties of iron can be changed if it is mixed with other substances.
ii) Copper loses its brown layer gradually when exposed to air.
Answer:
Copper reacts with moist carbon dioxide in the air and slowly loses its shiny brown surface and gains a green coat.
iii) Aluminium oxide is called amphoteric oxide.
Answer:
Aluminium oxide (Al2O3) reacts with both acid as well as bases to produce salt ans water.
![]()
Question 42.
(i) Write the differences between homologous organs and analogous organs.
Answer:
(i) Differences between homologous organs and analogous organs:
Homologous organs:
- Organs of different organisms have common origin
- They have similar structure and perform different function
- Ex: Forelimbs of frog and forelimbs of bird
Analogous organs:
- Organs of different organisms have different origin
- They have different structure and perform similar function
- Ex: Wings of bird and wings of bat.
(ii) Write the differences between the sex chromosomes of man and sex chromosomes of woman.
Answer:
Woman has a perfect pair of sex chromosomes, both called X. Man has a normal sized chromosome X and another short sized chromosome Y.
(iii) Sex of a child is determined by the father. How ?
Answer:
A child who inherits X chromosome from her father will be a girl and a child who inherits Y chromosome from his father will be a boy. Both the girl and the boy inherit only X choromosome from the mother. Therefore sex of a child is determined by the father.