Students can download Class 10 Science Chapter 3 Metals and Non-metals Important Questions, KSEEB SSLC Class 10 Science Important Questions and Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka SSLC Class 10 Science Important Questions Chapter 3 Metals and Non-metals
Question 1.
How are chemical elements classified on the basis of their chemical properties?
Answer:
Chemical elements are broadly classified on the basis of their chemical properties into two groups: metals and non-metals.
Question 2.
What are metals? Give examples.
Answer:
Metals are chemical elements that are opaque, ductile, lustrous and are good conductors of heat and electricity.
E.g.: Lead, sodium, silver, mercury, etc.
![]()
Question 3.
List the physical properties of metals.
Answer:
- Metals in pure form have a characteristic metallic lustre.
- Metals are generally hard. However, hardness varies from metal to metal.
- Metals are generally malleable. This means they can be beaten into thin sheets.
- Metals are usually ductile. This means most metals can be drawn into thin wires.
- Metals are good conductors of heat and electricity.
- Metals are generally sonorous. This means most metals produce a characteristic metallic sound on striking a hard surface.
- Most metals have high density, high melting point and high boiling point.
- All metals, except mercury, are solids at room temperature.
- Generally metals have high load-bearing capacity. This means they have high tensile strength.
Question 4.
- Show that metals in their pure form have a bright shiny surface.
- How can we show that metals are generally hard substances?
Answer:
1. Take a piece of iron. Note its appearance. It appears to have a dull surface. Clean the surface of the metal by rubbing it with sand paper. Now the metal gets a bright shiny appearance. Try this with other available metals such as magnesium, copper, aluminium etc.
We observe that the fresh surface of these metals has a unique bright, shiny appearance. This is known as metallic lustre. From this activity, we may conclude that pure metals usually have a characteristic metallic lustre.
2. Take small pieces of easily available metals. Try to cut each of these metals with a knife. It is observed that it is difficult to cut most metals with a knife. This activity shows that metals are generally hard substances.
Question 5.
Metals are usually hard substances, which cannot be easily cut with a knife. Name two metals that can be easily cut with a knife.
Answer:
Alkali metals like potassium and sodium can be easily cut into pieces with a knife.
![]()
Question 6.
What is malleability and ductility?
OR
Explain the meaning of the terms ‘malleable’ and ‘ductile’.
Answer:
1. Malleability:
The characteristic property of most metals that allows them to be bent and twisted into practically any shape without cracking or rupturing and of being beaten or rolled into thin sheets is known as malleability. Metals are said to be malleable because they can be beaten into thin sheets.
2. Ductility:
The characteristic property of most metals that allows them to be drawn into thin wires is known as ductility. Metals in general are said to be ductile because most metals can be drawn into thin wires.
Question 7.
Metals are usually malleable. What is the meaning of this statement?
Answer:
This statement means that most metals can be bent and twisted without breaking and they can be beaten into thin sheets.
Question 8.
Metals are usually ductile. What do you understand by this statement?
Answer:
This statement means that most metals can be drawn into thin wires.
Question 9.
How do you experimentally establish that metals are malleable?
Answer:
Malleability refers to the property of metals that enables them to be beaten into thin sheets. Take pieces of iron, zinc, lead and copper. Place any one metal on a block of iron and strike it four or five times with a hammer. The metal becomes thinner and wider. Similar effect is observed with most of the other metals. This shows that metals are usually malleable.
![]()
Question 10.
Name some of the metals that are usually used to make thin wires.
Answer:
Metals such as iron, copper, aluminium, silver, zinc and gold are used to make thin wires for various purposes.
Question 11.
Which is the metal that is most ductile? Justify your answer.
Answer:
Gold is the most ductile metal. A thin wire of about 2 km. length can be drawn from one gram of gold. This indicates the extent of its ductility.
Question 12.
What properties of metals enable them to be given any desired shape?
Answer:
High ductility and malleability of metals enable them to be given any desired shape.
Question 13.
How do you show that metals are good conductors of heat?
Answer:
Take a thin rod of aluminium. Clamp it on a stand. Fix a pin on the rod at its free end using wax. Heat the rod with a spirit lamp from the opposite end. After a while, the wax will melt and the pin will fall down. Try this activity using rods of different metals. It is observed that similar result is seen with most metals. This activity shows that metals in general are good conductors of heat.
Question 14.
Name two metals that are best conductors of heat.
Answer:
Metals that are best conductors of heat are silver and copper.
![]()
Question 15.
Show by an experiment that metals are good conductors of electricity.
Answer:
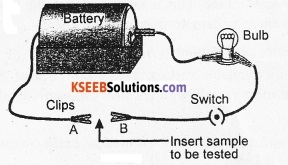
Set up an electric circuit with a battery, a switch and a bulb as shown in the figure. Place the metal to be tested in the circuit between terminals A and B connecting the two terminals. The bulb glows. Try with different metals. In each case, the bulb glows. This shows that metals are good conductors of electricity.
Question 16.
You are given a hammer, a battery, a bulb, wires and a switch.
- How could you use them to distinguish between samples of metals and non-metals?
- Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer:
1. A hammer can be used to beat the given substance. If the sample substance becomes thin and wide on hammering, it is a metal. If it crushes into a powder, it is a non-metal. We can connect the battery, bulb, switch and the given sample substance in series. If the circuit gets completed and the bulb glows, the sample substance is a metal. If the bulb does not glow, the sample substance is a non-metal.
2. Hammering is a more reliable method to distinguish between metals and non-metals. This is because no non-metal is malleable. The other test with battery and bulb can be less reliable because graphite (carbon, a non-metal) is a conductor of electricity.
Question 17.
Why are conducting wires given an insulating cover?
Answer:
Most electrical wires are covered with a rubber or plastic coating called insulation. The purpose of insulating cover over the metal part of an electrical wire is to prevent accidental contact with other conductors of electricity, which might result in an unintentional flow of electric current.
Question 18.
Give an example of a metal which
- Is a liquid at room temperature,
- Can be easily cut with a knife.
- Is the best conductor of heat,
- Is a poor conductor of heat.
Answer:
- The metal that is a liquid at room temperature is mercury.
- The meta! that can be easily cut with a knife is sodium.
- The metal that is the best conductor of heat is silver.
- A metal that is a poor conductor of heat is lead.
Question 19.
Why are metals said to be sonorous?
Answer:
Metals usually give a characteristic resonant ringing sound when hit by another object. This is why they are said to be sonorous.
![]()
Question 20.
What are non-metals? Give examples.
Answer:
Chemical elements that do not have the characteristic metallic properties like shining, hardness, thermal conductivity, electrical conductivity, malleability and ductility are called non-metals.
E.g.: Oxygen, bromine, iodine, helium, carbon etc.
Question 21.
Write the symbol, type of surface, hardness, malleability, ductility, thermal conductivity,
electrical conductivity and sonority of the following non-metallic elements: Graphite (carbon), sulphur and iodine.
Answer:
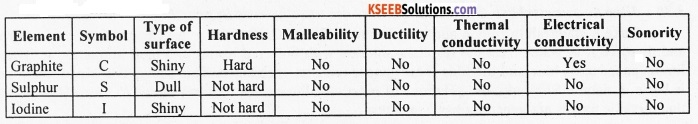
Question 22.
List the general characteristics of non-metals.
Answer:
- Non-metals are generally non-lustrous chemical elements.
- Some non-metals are in solid state, a few others are in liquid state and the rest are in gaseous state at room temperature.
- Non-metals have low density, low melting point and low boiling point.
- Non-metals are usually non-conductors of heat and electricity.
- They do not produce the characteristic metallic sound.
- They are neither malleable nor ductile; they are soft and brittle materials.
- The tensile strength of non-metals is very low.
Question 23.
Non-metals are usually non-lustrous. Name two non-metals that are lustrous.
Answer:
Graphite (carbon) and iodine are examples of non-metals that are lustrous.
![]()
Question 24.
Non-metals are usually non-conductors of electricity. Name a non-metal that conducts electricity.
Answer:
Graphite (carbon) is an example of a non-metal that conducts electricity.
Question 25.
Non-metals are usually soft and brittle. Give an example of a non-metal that is very hard.
Answer:
An example of a very hard non-metal is diamond (a form of carbon).
Question 26.
Name a non-metal that exists in liquid state at room temperature.
Answer:
A non-metal that exists in liquid state at room temperature is bromine.
Question 27.
Distinguish between the physical properties of metals and non-metals.
Answer:
| Metals | Non-metals |
| 1. All metals except mercury are solids at room temperature. Mercury is a liquid. | Non-metals occur in all the three states namely solid, liquid and gaseous state. |
| 2. Metals are generally sonorous. | Non-metals are non-sonorous. |
| 3. Metals are generally malleable and ductile. | Non-metals are brittle. They are neither malleable nor ductile. |
| 4. Metals are good conductors of heat and electricity. | Non-metals are bad conductors of heat and electricity. |
| 5. Metals have a characteristic metallic lustre. | Most non-metals are non-lustrous substances. |
Question 28.
Classify the following into metals and non-metals: mercury, gold, calcium, bromine, oxygen, sodium, sulphur and magnesium.
Answer:
- Metals: Mercury, gold, calcium, sodium, magnesium.
- Non-metals: Bromine, oxygen, sulphur.
![]()
Question 29.
What is allotropy? Name a non-metal that exhibits allotropy.
Answer:
The existence of a chemical element in more than one form with various forms having different physical properties but similar chemical properties is known as allotropy. Different forms of the same chemical element are called allotropes.
An example of a non-metal that exhibits allotropy is carbon. Two well-known allotropes of carbon are diamond and graphite.
Question 30.
How do you establish that metallic oxides are usually basic in nature?
Answer:
Take a magnesium ribbon and bum it over a flame. Collect the ashes (magnesium oxide) formed and dissolve it in water. Test the resultant solution with both red litmus and blue litmus paper. The red litmus paper turns blue and the blue litmus paper remains unchanged.
This shows that magnesium oxide is basic in nature. Similarly, the solutions of most metallic oxides turn red litmus to blue. This indicates that metallic oxides are generally basic in nature.
Question 31.
How do you experimentally establish that non-metallic oxides are usually acidic in nature?
Answer:
Take sulphur powder in a deflagrating spoon. Heat it over a flame. Sulphur combines with oxygen and forms sulphur dioxide in the form of a gas. Collect the gas in a test tube. Add about 3-4 mL of water to it and shake well.
Introduce both blue litmus and red litmus paper into the solution. The blue litmus turns red while the red litmus remains unchanged. This shows that sulphur dioxide is acidic in nature. Similarly, the solutions of most non-metallic oxides turn blue litmus to red. This indicates that non-metallic oxides are generally acidic in nature.
![]()
Question 32.
Pratyush took sulphur powder on a spatula and heated it. He collected the eas evolved by inverting a test tube over the burning sulphur.
- What will be the action of this gas on:
- Dry litmus paper?
- Moist litmus paper?
- Write a balanced chemical equation for the reaction taking place.
Answer:
- When sulphur is burnt in air, a gas called sulphur dioxide is formed.
- Sulphur dioxide gas has no action on dry litmus paper.
- Sulphur dioxide gas turns moist blue litmus paper to red.
- S (s) + O2 (g) → SO2 (g).
Question 33.
What happens when metals are burnt in air? Give two examples.
Answer:
Most metals react with and burn in air at various temperatures forming their respective metallic
oxides.
Metal + Oxygen → Metallic oxide
E.g.l: Aluminium bums in air on heating over a flame and forms aluminium oxide.
Aluminium (s) + Oxygen (g) → Aluminium oxide (s)
![]()
E.g.2: Copper on heating in air, combines with oxygen to form a black oxide called copper (II) oxide.
Copper (s) + Oxygen (g) → Copper oxide (s)
![]()
Question 34.
How do you show experimentally the reaction between a metal and oxygen?
Answer:
Hold a piece of magnesium ribbon with a pair of tongs. Heat the free end of the ribbon over a flame. Magnesium bums brilliantly producing a bright flame. In this process, magnesium combines with oxygen forming magnesium oxide, which appears in the form of ash.
![]()
![]()
Question 35.
Explain the reaction of aluminium metal with oxygen. Write a balanced equation for the reaction.
Answer:
Aluminium on heating over a flame burns in oxygen with a white flame to form aluminium oxide.
![]()
Question 36.
Give reason: Silver articles when exposed to air gradually turn blackish.
Answer:
Silver reacts with sulphur present in the air to form a coating of silver sulphide. This makes silver utensils black and dull when they are exposed to air.
Question 37.
The atomic numbers of two elements A and B are 11 and 12 respectively. Which element exhibits highest metallic property? Why? Write the molecular formula of the compounds formed when these elements combine with the element ‘Z’ having atomic number 8.
Answer:
Element A is sodium and element B is magnesium. Sodium exhibits highest metallic property, because the valence electrons in sodium are easier to be donated, hence contributing to high reactivity.
2 Na + O2 → 2 Na2O Element Z is oxygen.
2 Mg + O2 → 2 MgO.
Question 38.
Do all metals react with air in the same manner? Explain with suitable examples.
Answer:
No, all metals do not react in the same manner with air (oxygen). For example, metals like sodium react with air at room temperature itself. Some metals like magnesium require heating for the reaction to occur. Some other metals like copper require very strong heating before the reaction occurs. However, all metals react with oxygen to give their respective oxides.
![]()
Question 39.
Why is sodium kept immersed in kerosene oil?
Answer:
Sodium is a high reactive element. If it is kept in the open, it can explosively react with moist oxygen, catch fire and form sodium oxide. In order to prevent accidental damage due to such a reaction, sodium is kept immersed in kerosene oil as sodium does not react with kerosene.
Question 40.
What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer:
Oxides that react with both acids and bases to form salt and water are known as amphoteric oxides. Such oxides show both acidic and basic behaviour.
Examples: Lead oxide (PbO), Zinc oxide (ZnO) and Aluminium trioxide (Al2O3) etc.
Question 41.
Explain why aluminium oxide is called an amphoteric oxide. Give equations for the reactions.
Answer:
Oxides that show both acidic and basic nature are called amphoteric oxides. Such oxides react with both acids and bases to give a salt and water. Aluminium oxide reacts with acid and base and yields a salt and water. Therefore, aluminium oxide is called an amphoteric oxide.
1. Reaction of aluminium oxide with hydrochloric acid:
Aluminium oxide reacts with hydrochloric acid forming aluminium tri-chloride (a salt) and water.
Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O
2. Reaction of aluminium oxide with sodium hydroxide:
Aluminium oxide reacts with sodium hydroxide (base) forming sodium aluminate (a salt) and water.
Al2O3 + 2 NaOH → 2 NaAlO2 + H2O
![]()
Question 42.
What are alkalis? Give two examples.
Answer:
Metallic oxides are insoluble in water but some of these dissolve in water to form hydroxides known as alkalis. In other words, alkalis are the bases that dissolve in water.
E.g.1: Sodium oxide is a metallic oxide that is basic in nature. It reacts with water and forms an alkali called sodium hydroxide.
Na2O (s) + H2O (l) → 2 NaOH (aq)
E.g. 2: Potassium oxide is a metallic oxide that is basic in nature. It reacts with water and forms an alkali called potassium hydroxide.
K2O (S) + H2O (l) → 2 KOH (aq)
Question 43.
Do all metals react with oxygen at the same rate? Explain.
Answer:
No, all metals do not react with oxygen at the same rate. Different metals show different reactivities towards oxygen. Metals such as potassium and sodium are highly reactive metals. They react so vigorously with oxygen that they catch fire if kept in the open.
Some metals like magnesium, aluminium, zinc etc., are not as reactive as sodium and potassium. They combine with oxygen slowly at room temperature and form a layer of their respective oxides on their surface. This acts as a protective layer on the metal and prevents further reaction.
Iron does not burn on heating but iron filings burn vigorously when sprinkled on the flame. Copper does not burn in oxygen. However, on heating strongly, copper forms black coloured layer of copper (II) oxide.
Silver and gold are least reactive metals. They do not react with oxygen even at high temperatures. This shows that reactivity of metals with oxygen is different for different metals.
![]()
Question 44.
What happens when metals react with water? Give an example.
Answer:
Metals react with water and produce a metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal hydroxide. Thus the end product of the reaction of a metal with water is an alkali if the metallic oxide is soluble in water.
Question 45.
Write equations for the reactions of
- Iron with steam,
- Calcium with water and
- Potassium with water.
Answer:
1. Iron reacts with steam to form iron oxide (Fe304) and hydrogen gas.
3 Fe (s) + 4 H2O (g) → Fe3O4 (s) + 4 H2 (g)
2. Calcium reacts with water to form calcium hydroxide and hydrogen.
Ca (s) + 2 H2O (l) → Ca(OH)2 (aq) + H2 (g)
3. Potassium reacts with cold water violently and catches fire. Potassium hydroxide and hydrogen gas are the products in this reaction.
2 K (s) + 2 H2O (l) → 2 KOH (aq) + H2 (g)
![]()
Question 46.
Why does calcium float in water?
Answer:
Calcium reacts with water to form hydrogen gas. Although calcium is heavier than water, due to the sticking of the H2 gas bubbles on calcium metal surface, it starts floating.
Question 47.
Reaction of calcium with water is less violent. Give reason.
Answer:
Calcium reacts with water to form calcium hydroxide and hydrogen. The heat produced in this reaction is less, which is insufficient to burn the hydrogen gas that is formed. Hence, the reaction of calcium with water is less violent.
Question 48.
Draw a neat diagram to show the action of steam on iron wool. Name the gas produced during the reaction.
Answer:
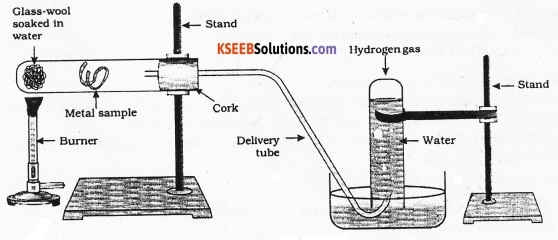
The gas produced when steam reacts with iron hydrogen.
![]()
Question 49.
How do you experimentally show that different metals react with water at different rates? Arrange these metals in the decreasing order of their reactivity.
Answer:
Collect small quantity of sodium, potassium, magnesium, calcium, copper and aluminium. Put a small piece of these metal samples separately in beakers half-filled with cold water. Sodium and potassium react vigorously and produce their respective hydroxides and hydrogen gas in the process. Potassium reacts more vigorously than sodium.
2 K (s) + 2 H2O (l) → 2 KOH (aq) + H2 (g)
2 Na (s) + 2 H2O (l) → 2 NaOH (aq) + H2 (g)
Calcium reacts slowly with cold water forming calcium hydroxide and hydrogen gas.
Ca (s) + 2 H2O (l) → Ca(OH)2 (aq) + H2 (g)
Magnesium and aluminium do not react with cold water.
Put the metals (Mg and Al) separately in beakers half-filled with hot water. Magnesium reacts with hot water forming magnesium hydroxide and hydrogen gas.
Mg + 2 H2O (l) → Mg(OH)2 (aq) + H2 (g)
Aluminium does not react even with hot water.
Aluminium, however, will react with steam forming aluminium oxide and hydrogen gas.
2 Al (s) + 3 H2O (g) → Al2O3 (S) + 3 H2 (g)
Copper does not react with water at all.
From this experiment we can conclude that, among the metals given, potassium is most reactive and copper is least reactive with water. Thus we can arrange these metals in the decreasing order of reactivity as: K > Na > Ca > Mg > Al > Cu.
Question 50.
Why does a piece of magnesium float in hot water but not in cold water?
Answer:
Magnesium does not react with cold water. Since magnesium is denser than water, it sinks in it. Magnesium however reacts with hot water and produces hydrogen gas. The bubbles of hydrogen gas stick to the metal and make it float in hot water.
Question 51.
How does iron react with water? Write a balanced chemical equation for the reaction.
Answer:
Iron does not react with either cold water or hot water. However, when steam is passed over red-hot iron, the reaction occurs resulting in iron oxide and hydrogen gas.
The equation for the reaction is:
3 Fe (s) + 4 H2O (g) → Fe3O4 (s) + 4 H2 (g).
![]()
Question 52.
Name four metals that do not react with water.
Answer:
Metals such as copper, silver, lead and gold do not react with water.
Question 53.
What products are formed when a metal reacts with mineral acids?
Answer:
Metals usually react with acids to give a salt of the metal and hydrogen gas.
Metal + Dilute acid → Salt + Hydrogen
However, all metals do not react with acids.
Question 54.
Different metals react with acids at different rates. IIow can you establish this fact? Arrange the metals in the decreasing order of their reactivity.
Answer:
Collect small quantity of pieces of magnesium, aluminium, zinc, copper and iron. Clean the surface of each of these metals with a sand paper. Put these metals in separate test tubes and place a thermometer in each of these test tubes. Add equal amount of dilute hydrochloric acid to each of the test tubes.
Observe the temperature in each test tube and also the rate of evolution of hydrogen gas. The rate of evolution of the gas is an indicator of the vigour of reactivity of the metal.
Magnesium is found to react most vigorously followed by aluminium, zinc, and then iron. Copper does not react with dilute hydrochloric acid.
The test tube with magnesium is found to be hottest while there is no change in temperature in the test tube containing copper. Thus we can arrange the given metals in the decreasing order of reactivity as: Mg > Al > Zn > Fe > Cu.
![]()
Question 55.
Write equations for the reactions of magnesium, aluminium, zinc and iron with dilute hydrochloric acid.
Answer:
Magnesium + dil. Hydrochloric acid → Magnesium chloride + Hydrogen gas
Mg (s) + 2 HCl (aq) → MgCl2 (aq) + H2 (g)
Aluminium + dil. Hydrochloric acid → Aluminium chloride + Hydrogen gas
2 Al (s) + 6HCl (aq) → 2 AlCl3 (aq) + 3H2 (g)
Zinc + dil. Hydrochloric acid → Zinc chloride + Hydrogen gas
Zn (s) + 2 HCl (aq) → ZnCl2 (aq) + H2 (g)
Iron + dil. Hydrochloric acid → Iron chloride + Hydrogen gas
Fe (s) + 2 HCl (aq) → FeCl2 (aq) + H2 (g)
Question 56.
What happens when magnesium (Mg) and manganese (Mn) react with very dilute nitric acid? Write equations for these reactions.
Answer:
Magnesium and manganese react vigorously with dilute nitric acid forming their respective nitrates along with hydrogen gas.
Magnesium + dil. Nitric acid → Magnesium nitrate + Hydrogen gas
Mg (s) + 2 HNO3 (aq) → Mg(NO3)2 (aq) + H2 (g)
Manganese + dil. Nitric acid → Manganese nitrate + Hydrogen gas
Mn (s) + 2 HNO3 (aq) → Mn(NO3)2 (aq) + H2(g)
Question 57.
Which gas is produced when dilute hydrochloric acid is added to a reactive metal?
Answer:
Hydrogen gas is produced when dilute HC1 is added to a reactive metal.
Question 58.
Write the chemical reaction when iron reacts with dilute H£Od.
Answer:
Fe (s) + H2SO4 (l) → FeSO4 (aq) + H2 (g).
![]()
Question 59.
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer:
Substances like lemon and tamarind contain acids. These acids dissolve the coating of copper oxide or copper carbonate formed on the surface of tarnished copper vessels. When these impurities get removed, copper vessels shine again in their original colour.
Question 60.
Name two metals that will displace hydrogen from dilute acids, and two metals that will not.
Answer:
Zinc and magnesium displace hydrogen from dilute acids. Gold and platinum do not displace hydrogen from dilute acids.
Question 61.
Why does dilute nitric acid react with magnesium and manganese and release hydrogen gas?
Answer:
Very dilute nitric acid reacts with magnesium and manganese and liberates hydrogen gas. The hydrogen is oxidised to water by nitric acid. Magnesium and manganese are active metals and are good reducing agents.
They quickly reduce water to hydrogen. This however does not happen with other metals. This is why dilute nitric acid releases hydrogen gas when it reacts with only manganese and magnesium and not with other metals.
Question 62.
Why can’t metals displace hydrogen from concentrated nitric acid?
Answer:
Concentrated nitric acid (HNO3) is a strong oxidising agent. It oxidises the hydrogen gas produced during the reaction to water. Nitric acid itself gets reduced to any of the nitrogen oxides (N2O, NO, or NO2). Therefore, hydrogen gas is not produced when a metal reacts with concentrated nitric acid.
![]()
Question 63.
How do you say that different metals have different reactivities? Explain with an example.
Answer:
To understand the fact that different metals have different reactivities, let us consider the reaction of different metals with dilute mineral acids. Copper does not displace hydrogen from dilute mineral acids.
Lead displaces hydrogen from dilute mineral acids very slowly. Iron displaces hydrogen from acids a little faster than lead but still slowly. Zinc reacts vigorously with dilute mineral acids and displaces hydrogen from them.
Sodium reacts violently with dilute mineral acids and displaces hydrogen from them. This shows that sodium is more reactive than zinc. Zinc is more reactive than iron. Iron is more reactive than lead and lead is more reactive than copper.
This establishes that different metals differ in their reactivities. We can write the reactivity series for these elements as follows: Na > Zn > Fe > Pb > Cu.
Question 64.
What is aqua regia? What is it used for?
Answer:
A freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3:1 by volume is called aqua regia. Aqua regia is a highly corrosive, fuming liquid. It is one of the few reagents used to dissolve gold and platinum.
Question 65.
Royal water is prepared by mixing two acids A and B. It can dissolve gold and platinum. It is a highly corrosive and fuming liquid. Identify A and B. What is the ratio in which A and B are mixed?
Answer:
Acid A is cone. HCl and B is cone. HNO3. Three parts of cone. HCl is mixed with one part of cone. HNO3 to make royal water called aqua regia.
![]()
Question 66.
A man went door-to-door posing as a goldsmith. He promised to bring back the slitter of old and dull sold ornaments. An unsuspecting lady save a set of sold bangles to him, which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Answer:
The person who was posing as goldsmith must have used Aqua regia. This is a mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio 3:1 by volume. Gold dissolves in aqua regia.
He must have used this solution to make bangles to sparkle like new ones. However, the weight of the bangles will have reduced drastically as some amount of gold dissolves in the solution.
Question 67.
How do metals react with solutions of other metal salts?
Answer:
More reactive metals displace less reactive metals from the solution of their salts. Less reactive metals, however, cannot displace more reactive metals from the solution of their salts.
Metal A (more reactive) + Salt solution of B → Salt solution of A + Metal B (less reactive)
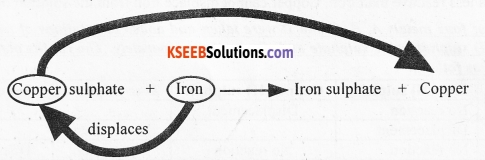
For example, iron is a more reactive metal than copper. Iron displaces copper from copper sulphate solution. However, copper cannot displace iron from iron sulphate.
![]()
Question 68.
Describe an experiment to show that a more reactive metal like iron can displace copper from a solution of copper sulphate but copper cannot displace iron from a solution of iron sulphate.
Answer:
Take a wire of copper and an iron nail. Clean their surface with sand paper. Put the copper wire in a solution of iron sulphate and the iron nail in a solution of copper sulphate taken in separate test tubes. Leave it for about 20 minutes. Now observe the copper wire and iron nail. Also observe the colour of the solutions in the two test tubes.
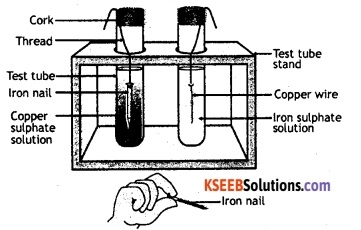
It is observed that a chemical reaction has taken place in the test tube in which iron nail was dipped in copper sulphate solution. The colour of the solution has changed from blue to green due to the formation of iron sulphate.
There are also brownish deposits of copper on the iron nail. Here, iron has displaced copper from the solution of copper sulphate. The reaction that has occurred in this test tube can be represented by the following equation:
CuSO4 (aq) + Fe (s) → FeSO4 (aq) + Cu (s).
There is no chemical reaction in the other test tube in which a copper wire was dipped in a solution of iron sulphate. Copper being a less reactive metal, cannot displace iron from a solution of iron sulphate.
Question 69.
Which metal, copper or iron, is more reactive? Give reason.
Answer:
Between copper and iron, iron is more reactive than copper. This is because iron can displace copper from a solution of its salt but copper cannot displace iron from a solution of its salt.
Question 70.
What would you observe when zinc is added to a solution of iron (II) sulphate? Write the chemical reaction that takes place.
Answer:
Zinc is more reactive (more electro positive) than iron. Therefore, it displaces iron from a solution of iron (II) sulphate and forms zinc sulphate. The iron (II) sulphate that was pale green will become colourless when zinc sulphate is formed.
FeSO4 (aq) + Zn (s) → ZnSO4 (aq) + Fe (s).
![]()
Question 71.
Give reason: Chemical reaction does not take place when copper is added to iron sulphate solution.
Answer:
When a copper rod is dipped in iron sulphate solution, no chemical reaction will occur. This is because copper is less reactive than iron. Copper cannot displace iron from the solution of its salt.
Question 72.
Samples of four metals A, B, C and D were taken and added to solutions of iron (II) sulphate, copper (II) sulphate, zinc sulphate and silver nitrate separately. The results obtained have been tabulated as follows:

Use the Table above to answer the following questions about metals A, B, C and D:
- Which is the most reactive metal?
- What would you observe if B is added to a solution of Copper (II) sulphate?
- Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer:
- B is the most reactive metal.
- B will displace copper from copper sulphate.
- Arrangement of the metals in the decreasing order of reactivity: B > A > C > D.
Question 73.
You are given a few grains of magnesium, iron and copper. Which of these metals
- does not displace hydrogen from the acid,
- forms a pale green compound,
- will give hydrogen gas with dilute nitric acid,
- will be displaced from a solution of its salt by the other metals?
Answer:
- Copper does not displace hydrogen from the acid.
- Iron forms a pale green compound.
- Magnesium gives hydrogen gas with dilute nitric acid.
- Copper will be displaced from a solution of its salt by other metals.
![]()
Question 74.
There are two metals X and Y. The following were observed with regard to these metals:
X + YSO4 → XSO4 + Y
Y + XSO4 → No reaction.
Out of these two metals, which is more reactive? Why?
Answer:
The data above show that X is a more reactive metal than Y. This is because X has replaced Y from a solution of its salt while Y could not displace X from its salt.
Question 75.
State which of the following reactions are possible giving reasons for your answer:
- Zn + CuSO4 → ZnSO4 + Cu
- Fe + ZnSO4 → FeSO4 + Zn
- Zn + FeSO4 → ZnSO4 + Fe
Answer:
- Reaction shown in 1 is possible because Zn is more reactive than copper. In the reactivity series Zn is placed above Cu.
- Reaction shown in 2 is not possible because Fe is less reactive than Zn. Zinc is placed above Fe in the reactivity series
- Reaction shown in 3 is possible because Zn is more reactive than Fe. We find Zn placed above Fe in the reactivity series.
Question 76.
What is reactivity series of metals? On what basis are the metals arranged in the reactivity series?
Answer:
A list of metals arranged in the order of their decreasing reactivity (activity) is called reactivity series. Metals are arranged in the reactivity series on the basis of displacement experiments performed on them.
Question 77.
Write the reactivity series of the commonly known metals such as Mg, Pb, Cu, Sn, Na, K, Au, Ag, Ca, Zn, Hg, Fe, Al, and Pb. From this series, state the least reactive and most reactive metal.
Answer:
Reactivity series of metals:
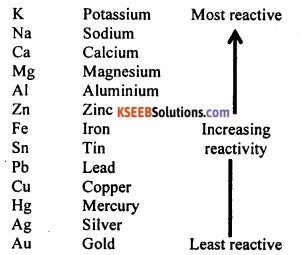
From the reactivity series, it is clear that gold (Au) is the least reactive metal and potassium (K) is the most reactive metal.
![]()
Question 78.
Name two metals that are found in nature in the free state. Why do they exist in nature in free state?
Answer:
Two metals that are found in nature in free state are platinum and gold. These metals are least reactive and hence are not easily attacked by air, water, acids, alkalis or other chemicals. Therefore, they exist in nature in free state.
Question 79.
How do you explain the reactivity of chemical elements?
Answer:
Elements that have either two or eight electrons in the outermost shell of their atoms are known to be highly stable. Such atoms have completely filled valence shell. Those elements whose atoms do not have this structure have a tendency to attain this arrangement either by transferring or sharing electrons with other atoms.
Those atoms that have completely filled valence shell are known to be least reactive. Those with incompletely filled valence shell are known to be chemically active. Therefore, we can explain the reactivity of elements on the basis of their tendency to attain a completely filled valence shell.
Question 80.
Metallic oxides of zinc, magnesium and copper were heated with the following metals: Zn, Ms and Cu. In which cases will you find displacement reactions taking place?
Answer:
On the basis of reactivity series of metals, the displacement reaction will take place as indicated in the table below:
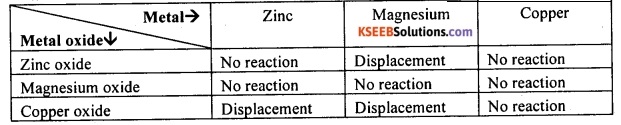
![]()
Question 81.
Give reason why copper is used to make hot water tanks and not steel (an alloy of iron).
Answer:
Copper is used to make hot water tanks and not steel. This is because the iron (in steel) is more reactive than copper. Iron reacts with hot steam to produce an oxide of iron. This makes the body of the tank weaker and weaker and this can be dangerous. Copper, however, does not react with hot water and hence is preferred in making hot water tanks.
Question 82.
Slate the differences in chemical properties of metals and non-metals.
OR
Differentiate between metals and non-metals on the basis of their chemical properties.
Answer:
| Chemical properties of Metals | Chemical properties of Non-metals |
| 1. Metals are electron donors and hence are electropositive. | Non-metals are electron acceptors and hence are electronegative. |
| 2. Generally, metals form ionic bonds. | Generally, form both ionic and covalent bonds. |
| 3. Generally, metals displace hydrogen from dilute acids. | Non-metals do not displace hydrogen from dilute acids. |
| 4. Aqueous solutions of metallic oxides turn red litmus blue. This means oxides of metals are basic in nature. | Aqueous solutions of non-metallic oxides turn blue litmus red. This means the oxides of non-metals are acidic in nature. |
| 5. Some oxides of metals in aqueous solution react with zinc to produce hydrogen. | Aqueous solutions of oxides of non-metals react with carbonates to produce carbon dioxide. |
Question 83.
What type of oxides are formed when non-metals combine with oxygen?
Answer:
Non-metals combine with O2 to form either
- Acidic oxides, e.g., CO2, SO2 etc.,
- Neutral oxides, e.g., N2O, H2O etc.
![]()
Question 84.
What is meant by electronic configuration of an element?
Answer:
The arrangement or distribution of electrons in the various shells and orbitals of an atom is called its electronic configuration. Electrons are filled in such a way that they achieve a high stable configuration.
Question 85.
Write the electronic configuration of helium, neon, argon, sodium, magnesium, calcium, oxygen, nitrogen, chlorine, phosphorus and sulphur indicating the number of electrons in each of their atoms. Which of these elements have the most stable structure? why?
Answer:
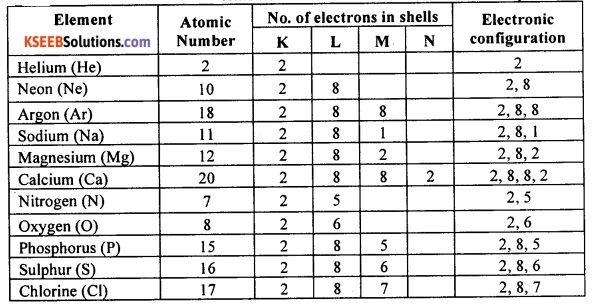
Among the elements given, helium, neon and argon have the most stable structure. This is because these elements have atoms with completely filled valence shell. Other elements have relatively less stable structure because they have incompletely filled valence shell.
![]()
Question 86.
What is octet arrangement or octet configuration?
Answer:
The arrangement of having eight electrons in the outermost shell of an atom is called octet arrangement or octet configuration. All noble gases except helium show this arrangement. This is why it is called noble gas structure. This structure is relatively more stable than others.
Question 87.
What is electron-dot structure? How is it represented?
Answer:
Electron-dot structure is a structural representation of a molecule where dots are used to show electron position around the atoms and lines or dot pairs represent covalent bonds between atoms. It is a pictorial representation of the valence electron configuration in dots around an atom.
Electron-dot structure is based on the concept of the octet rule in which atoms share electrons so that each atom has 8 electrons in its outermost shell.
Question 88.
Represent the ionization of sodium atom into sodium ion using electronic-dot structure. Explain the process.
Answer:
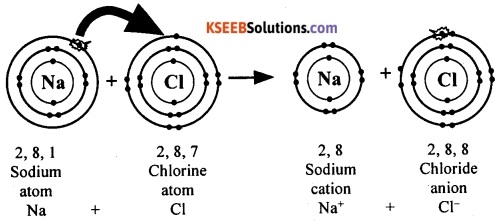
The electronic configuration of sodium atom is 2, 8, 1. This means an atom of sodium has one electron in its outermost shell (M shell). If it loses the electron from its M shell, its L shell will now become the outermost shell and that has a stable octet.
Now, the electronic configuration becomes 2, 8. The nucleus of this atom still has 11 protons but the number of electrons has become 10. Thus, by losing an electron, the sodium atom acquires a net positive charge and becomes sodium ion (Na+).

![]()
Question 89.
Represent the ionization of chlorine atom into chloride ion using electron-dot structure. Explain the process.
Answer:

The electronic configuration of chlorine atom is 2, 8, 7. This means an atom of chlorine has seven electrons in its outermost shell (M shell). If it gains one electron, the M shell will get a total of 8 electrons that has a stable octet structure.
Now, the electronic configuration becomes 2, 8, 8. The nucleus of this atom still has 17 protons but the number of electrons has become 18. Thus, by gaining an electron, the chlorine atom acquires a net negative charge and becomes chloride ion (Cl–).
Question 90.
Explain the bond formation in sodium chloride.
Answer:
The electronic configuration of sodium is 2, 8, 1. It has just one electron in its valence shell. It should either lose one electron or gain seven electrons to obtain octet structure. It is easy for an atom of sodium to lose an electron and to become Na+ ion.
An atom of chlorine has seven electrons in its valence shell. It should either lose seven electrons or gain one electron to attain the octet structure. It is easy for a chlorine atom to gain an electron and to become Cl– ion.
When sodium and chlorine come together, an electron from the valence shell of sodium is transferred to the valence shell of chlorine atom. Thus an electrostatic force of attraction binds the two atoms by an ionic bond. Thus, sodium chloride is formed by ionic bond between the atoms of sodium and chlorine.
![]()
Question 91.
Sodium and chlorine are placed in the third period of the modern periodic table in groups 1 and 17 respectively. State their valency. Which of them can form anion? Which of them can form cation? Give reason for your answer.
Answer:
The atomic number of sodium is 11 and the electronic configuration of sodium is 2, 8, 1. The valency of sodium is 1. The atomic number of chlorine is 17 and its electronic configuration is 2, 8, 7.
The valency of chlorine is also 1. Sodium atom forms a cation (positive ion) by giving up its lone electron in the valence shell in order to attain the octet structure. Chlorine atom forms an anion (negative ion) by receiving 7 electrons from some other atom in order to attain the octet structure.
Question 92.
Represent the formation of sodium chloride from sodium atom and chlorine atom using electron-dot structure.
Answer:
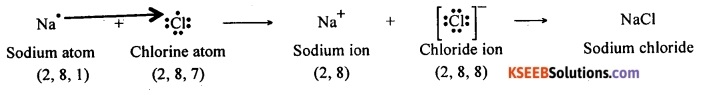
Question 93.
Represent the formation of sodium chloride from sodium atom and chlorine atom using electron-dot structure. Explain the process.
Answer:

The electronic configuration of magnesium atom is 2, 8, 2. This means an atom of magnesium has two electrons in its outermost shell (M shell). If it loses the two electrons from its M shell, its L shell will now become the outermost shell and that has a stable octet.
Now, the electronic configuration becomes 2, 8. The nucleus of this atom still has 12 protons but the number of electrons has become 10. Thus by losing two electrons, the magnesium atom acquires a net positive charge and becomes magnesium ion (Mg++).
![]()
Question 94.
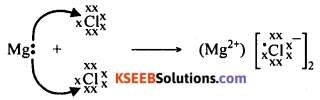
Explain the bond formation in magnesium chloride.
Answer:
The electronic configuration of magnesium is 2, 8, 2. It has two electrons in its valence shell (M shell). It should either lose two electrons or gain six electrons to attain the stable octet structure. It is easy for an atom of magnesium to lose two electrons to attain the stable octet structure and to become Mg++ ion.
An atom of chlorine has seven electrons in its valence shell. It should either lose seven electrons or gain one electron to attain the stable octet structure. It is easy for chlorine atom to gain an electron and to become Cl– ion.
When an atom of magnesium and two chlorine atoms come together, one electron from the valence shell of magnesium is transferred to the valence shell of one of the chlorine atoms and another electron to the valence shell of the other chlorine atom.
Thus an electrostatic force of attraction binds the two atoms by an ionic bond. Thus, magnesium chloride is formed by ionic bond between the atoms of magnesium and chlorine.
Question 95.
- Write the electron-dot structures for sodium, oxygen and magnesium.
- Show the formation of Na2O and MgO by the transfer of electrons.
- What are the ions present in these compounds?
Answer:
1. Electron-dot structure of sodium, oxygen and magnesium:

2. Formation of sodium oxide from sodium and oxygen by electron transfer:

Formation of magnesium oxide from magnesium and oxygen by electron transfer:
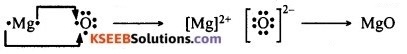
3. The ions present in sodium oxide are Na+ and O2- (also written as O<sup–) and the ions present in magnesium oxide are Mg2+ and O2- (also written as O—).
![]()
Question 96.
Draw a neat diagram to show testing of the conductivity of a salt solution.
Answer:
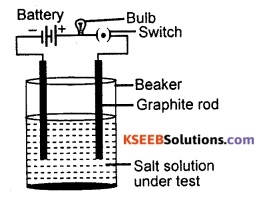
Question 97.
What is an ionic bond? Explain with an example.
Answer:
A chemical bond formed between two atoms due to the transfer of one or more electrons from the outermost shell of one of those atoms to the outermost shell of the other atom is called an ionic bond.
The bond formed between the sodium atom and chlorine atom in sodium chloride is an example of an ionic bond. During bond formation, an electron from sodium atom is transferred to the chlorine atom.
Sodium atom becomes a cation on losing an electron. Chlorine atom that gains an electron becomes an anion. The two ions attract each other resulting in the formation of an ionic bond between them.
Question 98.
What are ionic compounds? Give two examples.
Answer:
Compounds that contain ions and are held together by ionic bonds are called ionic compounds. They are chemical compounds formed by the electrostatic attraction between two oppositely charged ions. They are also known as electrovalent compounds. When a metal is combined with one or more non-metals, the compound is usually ionic.
Examples: Sodium chloride, copper sulphate, magnesium oxide, potassium iodide etc.
![]()
Question 99.
Write the properties of ionic compounds.
Answer:
The following are some of the general properties of ionic compounds:
- All ionic compounds are hard solids.
- Ionic compounds are found in crystal form rather than in amorphous form.
- Ionic compounds are brittle and break into pieces when pressure is applied.
- They have high melting and boiling points.
- They are usually soluble in water but are insoluble in solvents.
- They conduct electricity in molten or solution state. However, they do not conduct electricity while in solid state.
Question 100.
Why do ionic compounds have high melting points?
Answer:
Ionic compounds are formed by the chemical union of positive and negative ions. These ions are bound strongly by the electrostatic force of attraction between the oppositely charged ions. Hence, a lot of heat is required to break this force of attraction and melt the ionic compounds. This is why ionic compounds have high melting points.
Question 101.
Why are ionic compounds hard solids?
Answer:
Ionic compounds are solids and are somewhat hard because of the strong force of attraction between the positive and negative ions, which make up their molecules.
Question 102.
Name one properly that is not shown by ionic compounds.
Answer:
Ionic compounds do not conduct electricity in the solid state.
![]()
Question 103.
Why do ionic compounds conduct electricity only in molten or solution stcte but not in solid state?
OR
Give reason: Ionic compounds in solid state do not conduct electricity, whereas in molten state are good conductors of electricity.
Answer:
Ionic compounds dissociate into positively charged anions and negatively charged cations in their molten or dissolved state and become free to move. Therefore, ionic compounds contain plenty of free ions in their molten state or dissolved state, which is why they are able to conduct electric current in that state.
When ionic compounds are in solid state, the ions are not free to move as they are held together by strong electrostatic force. This is why ionic compounds cannot conduct electricity when they are in solid state.
Question 104.
What are minerals?
Answer;
Elements or inorganic compounds of metals that occur naturally in the earth’s crust are called minerals.
Question 105.
“Every mineral has a definite and a fixed composition”. Explain.
Answer:
Minerals are chemical compounds that are widely distributed in the earth’s crust in the form of carbonates, oxides, sulphates, sulphides, nitrates, etc. All chemical compounds have chemical elements in a fixed composition. They may contain impurities, which can be physically separated by employing a suitable method. However, the chemical composition of every mineral is definite and fixed.
![]()
Question 106.
What are ores?
Answer:
Minerals from which metals can be extracted profitably and conveniently are called ores.
Question 107.
How does the term ‘ore’ differ from ‘mineral’? Explain with an example.
Answer:
Minerals are naturally occurring chemical compounds of a metal, which may be found along with more impurities. But ore is a chosen mineral of a metal, from which metal is extracted profitably on a large scale, in pure form.
For example, both haematite and iron pyrites are minerals of iron. However, it is not economical to produce iron from iron pyrites. Iron can be extracted from haematite conveniently and profitably. Therefore haematite is an ore of iron and iron pyrites is just a mineral.
Question 108.
What are the various forms in which metal ores are available in nature?
Answer:
Metal ores are found in nature in the form of oxides, sulphides, carbonates, chlorides, fluorides or phosphates.
Question 109.
All ores are minerals but all minerals are not ores. Explain this statement.
Answer:
A particular metal may be present in several minerals. However, it may not be convenient or profitable to extract the metal from all these minerals. Only that mineral from which a metal is extracted conveniently and economically is called an ore. Therefore, all ores are minerals but all minerals are not ores.
![]()
Question 110.
Define the following terms:
- Mineral
- Ore
- Ganeue.
Answer:
1. Mineral:
Naturally occurring compounds of elements found in the earth’s crust are known as minerals.
2. Ore:
Any mineral from which a metal can be profitably extracted is called an ore of that metal.
3. Gangue:
Impurities like sand, rock etc., which are present in an ore, are known as gangue materials.
Question 111.
How do metals occur in nature?
Answer:
Some metals are found in the earth’s crust in the free state. Many metals are found in the form of their compounds such as oxides, sulphides, chlorides, phosphates, carbonates or fluorides. The metals at the bottom of the activity series are the least reactive. They are often found in a free state.
For example, gold arid platinum are found in the free state. Copper and silver are also found both in free state and combined state. The metals at the top of the activity series (K, Na, Ca, Mg and Al) are so reactive that they are never found in nature as free elements.
The metals in the middle of the activity series (Zn, Fe, Pb, etc.) are moderately reactive. They are found in the earth’s crust mainly as oxides, sulphides or carbonates. You will find that the ores of many metals are oxides. This is because oxygen is a very reactive element and is very abundant on the earth.
Question 112.
On the basis of their chemical reactivity, how are metals classified? Why is such a classification important?
Answer:
On the basis of reactivity, metals are classified into three groups:
- Metals of low reactivity,
- Metals of medium reactivity, and
- Metals of high reactivity.
This type of classification of metals is important because different techniques are to be used for obtaining the metals falling in each of these categories.
![]()
Question 113.
Give two examples each of metals of low reactivity, metals of medium reactivity and metals of high reactivity.
Answer:
Metals of low reactivity include mercury and copper. Examples of metals of medium reactivity include zinc and aluminium. Examples of metals of high reactivity include potassium and sodium.
Question 114.
Represent by a flow diagram the steps involved in the extraction of metals of various degrees of reactivity from their respective ores.
Answer:
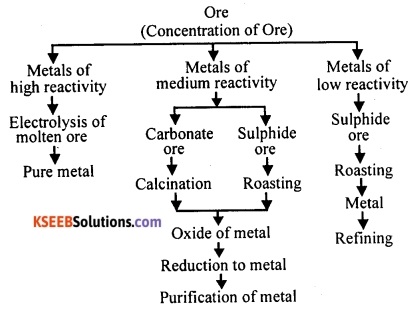
Question 115.
Define metallurgy.
Answer:
The process of extracting metals from their ores and subsequently refining them is called metallurgy.
Question 116.
What are the three major steps involved in extraction of a metal from its ore?
Answer:
The three major steps involved in extraction of a metal from its ore are:
- Concentration of the ore to remove impurities,
- Reduction of the ore to get the metal, and
- Purification of the ore.
Question 117.
What is meant by concentration of an ore? Why is it done?
Answer:
Naturally occurring ores usually contain many impurities such as sand, rocks etc. These impurities are known as gangue materials. The process of removing the gangue materials present in an ore by employing one or the other physical methods is called concentration of the ore.
This is also called enrichment of the ore. Concentration of the ore is done to remove undesirable impurities present in the ore.
![]()
Question 118.
On what factor does the method employed for the enrichment of the ore depend?
Answer:
The technique or the process used for removing the gangue from the ore depends on the differences between the physical or chemical properties of the gangue and the ore.
Question 119.
What chemical process is used for obtainine a metal from its oxide?
Answer:
A metal is extracted from its oxide ore by the process of reduction. The oxide is reduced to metal form by using a suitable reducing agent.
Question 120.
Explain with an example how a metal of low reactivity is extracted from its ore.
Answer:
Mere heating can reduce the oxides of metals of low reactivity. No other reducing agent is necessary to obtain the metal from its oxide. Let us understand this with an example. Mercury (Hg) is a metal of low reactivity. Its commonly known ore is cinnabar (HgS). It is a sulphide ore.
First, the ore is concentrated by a suitable process. The concentrated cinnabar is converted into oxide form by heating it in air.
Mercuric sulphide + Oxygen → Mercuric oxide + Sulphur dioxide
![]()
Mercuric oxide gets reduced to mercury on further heating in absence of air.
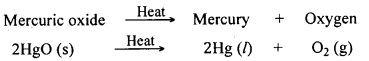
![]()
Question 121.
A metal that exists as a liquid at room temperature is obtained by heating its sulphide in the presence of air. Identify the metal and its ore and give the reaction involved.
Answer:
Mercury is the only metal that exists as a liquid at room temperature. Its ore is cinnabar (HgS). Reactions involved are:
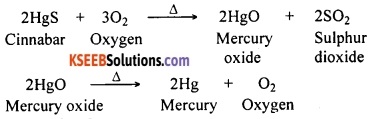
Question 122.
A sample of an ore is heated in a test tube. In the beginning a gas is evolved which turns wet blue litmus red. On further heating, a gas is evolved which makes a glowing splinter burn brilliantly.
A shining greyish metal appears in the test tube. Study the data and answer the following questions:
- What are the two gases produced?
- What is the possible name of the ore?
- Which is the metal produced in the test tube?
Answer:
- The two gases produced here are sulphur dioxide and oxygen.
- The ore in the test tube is most likely to be Cinnabar (an ore of mercury).
- The metal left behind in the test tube is mercury.
Question 123.
What is roasting in metallurgy?
Answer:
In metallurgy, heating a sulphide ore in presence of air (oxygen) is called roasting.
![]()
Question 124.
Briefly explain how a low reactivity metal like copper is extracted from copper glance.
Answer:
Copper is a metal of low reactivity. Its sulphide ore is called copper glance (Cu2S). First, the ore is concentrated by a suitable process. The concentrated copper glance is first converted into oxide form by heating it in air.
![]()
Copper oxide so formed reacts with copper sulphide (the ore itself) and gets reduced to copper on further heating in absence of air.
![]()
The copper so obtained is purified by electrolytic refining.
Question 125.
Why are sulphide and carbonate ores of metals first converted into oxide form while extracting the metal?
Answer:
It is easier to obtain a metal from its oxide, as compared to its sulphides and carbonates. Therefore, prior to reduction, the metal sulphides and metal carbonates are first converted into their respective metal oxides.
Question 126.
Give some examples of metals with medium reactivity. Where are these metals placed in the reactivity series?
Answer:
Metals with medium reactivity include zinc, iron, lead and manganese. They are placed in the middle of the reactivity series.
Question 127.
Name the gaseous products obtained by the electrolysis of aqueous sodium chloride solution.
Answer:
The gaseous product formed during the electrolytic reduction of aqueous sodium chloride solution is chlorine.
![]()
Question 128.
Give the differences between roasting and calcination with suitable examples.
Answer:
| Roasting | Calcination |
| 1. Ore is heated in excess of air. | Ore is heated in the absence or limited supply of air. |
| 2. This is used for sulphide ores. | This is used for carbonate ores. |
| 3. SO2 is produced along with metal oxide. | CO2 is produced along with metal oxide. |
Question 129.
Explain how a metal of medium reactivity such as zinc is extracted from its sulphide ore.
Answer:
Zinc is a metal of medium reactivity. The commonly known ore of zinc is zinc sulphide (ZnS). The ore is first concentrated by a suitable technique. The concentrated ore is heated strongly in presence of air. This process is known as roasting. Zinc sulphide on roasting yields zinc oxide and sulphur dioxide.
![]()
The zinc oxide so obtained is heated strongly with carbon (coke) in absence of air. Carbon acts as a reducing agent. It reduces zinc oxide to zinc metal.
![]()
The metal so obtained is refined to get pure zinc.
Question 130.
Explain with suitable equations how zinc is obtainedfrom its carbonate ore.
Answer:
The carbonate ore of zinc is called Smithsonite (ZnCO3). The ore is first concentrated by a suitable technique. The concentrated ore is heated strongly in limited supply of air. This process is known as calcination. Zinc carbonate on calcination decomposes to zinc oxide and carbon dioxide.
![]()
The zinc oxide so obtained is heated strongly with carbon (coke) in absence of air. Carbon acts as a reducing agent. It reduces zinc oxide to zinc metal.
![]()
The metal so obtained is refined to get pure zinc.
![]()
Question 131.
Give an example to show that metals can be used as reducing agents to convert oxide ores of metals.
Answer:
Highly reactive metals such as sodium, calcium, aluminium, etc., are sometimes used as reducing agents because they can displace metals of lower reactivity from their compounds.
For example, when manganese dioxide is heated with aluminium powder, manganese dioxide is reduced to metallic manganese while aluminium gets oxidized to aluminium trioxide.
3 MnO2 (s) + 4Al (s) → 3 Mn (l) + 2 Al2O3 (s) + Heat
This reaction is highly exothermic as it produces a large amount of heat.
Question 132.
What are thermit reactions? Give an example.
Answer:
A highly exothermic chemical reaction in which a metallic oxide reacts with aluminium and gets reduced to metallic form is known as thermit reaction.
For example, the reaction of iron (III) oxide (Fe2O3) with aluminium yields aluminium oxide, metal iron and plenty of heat. Therefore, this is a thermit reaction.
Fe2O3 (s) + 2Al (s) → 2Fe (l) + Al2O3 (s) + Heat.
Question 133.
List the characteristics of thermit process.
Answer:
The following are the characteristics of thermit process:
- These are highly exothermic reactions, which produce a very large amount of heat.
- In such reactions, highly reactive metals such as sodium, calcium, aluminium etc., act as reducing agents.
- The heat produced in these reactions is so large that the metals are produced in molten state.
![]()
Question 134.
List any two uses of thermit reactions.
Answer:
Thermit reactions are used:
- To join railway tracks.
- To join cracked machine parts.
Question 135.
The reaction of iron (III) oxide (Fe2O3) with aluminium is used to join railway tracks or to join cracked machine parts. Give reason.
Answer:
The reaction between iron (III) oxide (Fe2O3) and aluminium is highly exothermic. This reaction releases so much heat that iron formed during the process is in molten state. This is why the reaction is used to join railway tracks or to join cracked machine parts.
Question 136.
Explain with an example the extraction of a highly reactive metal from its ore.
Answer:
Highly reactive metals cannot be obtained from their compounds by reducing them with carbon (coke). This is because these metals have greater affinity for oxygen than carbon. Such metals are obtained by electrolytic reduction of their compounds.
For example, sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides. The metals are deposited at the cathode (the negatively charged electrode). Chlorine is liberated at the anode (the positively charged electrode). The reactions are as follows:
At cathode: Na+ + e– → Na
At anode: 2Cl– → Cl2 + 2e–
Similarly, aluminium is obtained by the electrolytic reduction of aluminium oxide.
![]()
Question 137.
What is meant by electrolytic reductioti? How is sodium obtained from its molten chloride? Explain.
Answer:
In electrolytic reduction, the metals are extracted by the electrolysis of their salts.
Sodium is obtained by the electrolysis of its molten chloride. The metals are deposited at the cathode (the negatively charged electrode), whereas chlorine is liberated at the anode (the positively charged electrode).
Reaction: NaCl \(\rightleftarrows\) Na+ + Cl–
At cathode: Na+ + e– → Na
At anode: 2Cl– → Cl2 + 2e–
Question 138.
In metallurgy, what is refining of a metal?
Answer:
The metals obtained through metallurgical operations are often impure. The process of purifying an impure metal using a suitable technique is known as refining.
Question 139.
How are metals such as copper, zinc, tin, nickel, silver, gold etc., obtained in metallurgical operations refined?
Answer:
Metals such as copper, zinc, tin, nickel, silver, gold etc., obtained in metallurgical operations are refined by using the technique of electrolysis. This is known as electrolytic refining.
Question 140.
Explain how a metal is refined by using the technique of electrolysis.
Answer:
A rod of impure metal is made the anode and a thin rod of pure metal is made the cathode. The two are dipped in a solution of a suitable metal salt. This solution acts as the electrolyte.
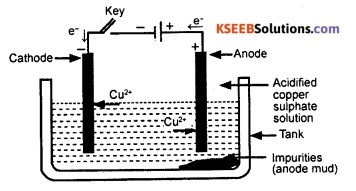
On passing current through the electrolyte, pure metal gets deposited on the cathode. The soluble impurities go into the solution, whereas, the insoluble impurities settle down at the bottom of the anode. This impurity that settles down at the bottom of the anode is known as anode mud.
![]()
Question 141.
What is anode mud?
Answer:
The impurity collected below the anode during electrolytic refining of metals is called anode mud.
Question 142.
Draw a neat, labelled diagram showing the electrolytic refining of copper.
Answer:
Question 143.
In the electrolytic refinine of a metal M, what would you take as the anode, the cathode and the electrolyte?
Answer:
In the electrolytic refining of a metal M, a rod of impure metal M is taken as anode. A wire of pure metal M is taken as cathode. The solution of a suitable salt of metal M is taken as the electrolyte.
Question 144.
What is corrosion? Explain with examples.
Answer:
The natural process in which gradual destruction of materials (usually metals) occurs due to attack by air, water, acids, bases and other chemicals present in the environment is known as corrosion.
For example, silver articles become black after sometime when exposed to air. This is because they react with sulphur in the air to form a coating of silver sulphide. Rusting of iron when it is exposed to atmospheric air is another example of corrosion of metals.
![]()
Question 145.
Which metals do not corrode easily? Give reason.
Answer:
Metals that are placed at the bottom of activity series like silver, gold and platinum do not corrode easily. This is because, they are least reactive and are not acted upon by air, water or acids.
Question 146.
Copper articles after sometime lose their shiny brown surface and gain a green coat. Give reason.
Answer:
Copper, when kept in the open, slowly reacts with moist carbon dioxide in the air and forms a greenish compound called copper carbonate. This is why copper articles lose their shiny brown surface and develop a green coat over them after sometime. This is an example of corrosion.
Question 147.
Silver metal does not combine easily with oxygen but silver articles become dark after sometime. Give reasons.
Answer:
Silver is a highly unreactive metal, so it does not react with the oxygen of air easily. But silver articles turn black after some time because they react with sulphur in the air to form a coating of silver sulphide.
Question 148.
How does iron react with air?
Answer:
Iron reacts with moist air forming brownish oxide of iron called rust.
Question 149.
What is rust? Give its chemical formula.
Answer:
A brownish hydrated oxide of iron that is formed on the surface of iron when it is exposed to moist air is called rust.
The chemical formula of rust is Fe2O3.2 H2O.
![]()
Question 150.
Explain the reaction of iron with oxygen. Write the word equation for this reaction.
Answer:
Iron combines with oxygen in presence of water vapour to form a hydrated oxide of iron called rust.
Iron + water + oxygen → hydrated iron oxide.
Question 151.
Which are the two substances essential for rusting of iron?
Answer:
The two substances essential for rusting of iron are oxygen (air) and water.
Question 152.
Show that air and moisture are essential for rusting of iron with the help of an experiment.
Answer:
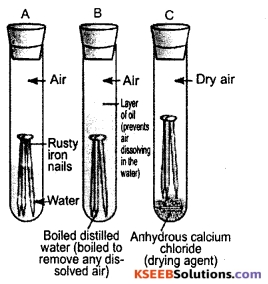
Take three test tubes. Place 2-3 cleaned iron nails in each of the test tubes. Label these test tubes A, B and C. Pour some water in test tube A and cork it. Pour boiled distilled water in test tube B, add about 1 mL of oil and cork it.
The oil will float on water and prevent the air from dissolving in the water. Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture, if any, present in the air. Iron in test tube A gets both air and water. Iron in test tube B gets only water but not air.
Iron in test tube C gets only air but not moisture. Leave these test tubes for a few days. After a few days it is observed that the iron nails in test tube A are rusted while those in B and C are not rusted. This shows that both air and moisture (water) are essential for rusting.
![]()
Question 153.
Mention some ways of preventing rusting of iron.
Answer:
Painting, oiling, greasing, galvanising, chrome plating, anodising or making alloys can prevent rusting of iron.
Question 154.
Iron nail kept in a closed jar containing calcium chloride does not rust. Give reason.
Answer:
Iron reacts with oxygen and rusts only in presence of moisture or water. Calcium chloride absorbs moisture and keeps the air inside the jar dry. Therefore, iron nail kept in a closed jar containing calcium chloride does not rust.
Question 155.
What is galvanisation? How does it help to prevent rusting of iron?
Answer:
The process of coating articles of steel or iron with a thin layer of zinc in order to prevent rusting is known as galvanisation.
Zinc coating prevents iron articles from coming into contact with corrosive substances and chemicals. Secondly, zinc is more reactive than iron. It preferentially combines with oxygen and prevents rusting.
Question 156.
A galvanised article is protected against rusting even if the zinc coating is broken. How is this possible?
Answer:
A galvanised article is protected against rusting even if the zinc coating is broken. This is because zinc is more reactive than iron. When the zinc coating over iron is broken, zinc readily reacts with oxygen and prevents iron from coming into contact with air. Thus zinc prevents rusting.
![]()
Question 157.
State two ways to prevent rusting of iron.
Answer:
- Iron objects can be protected from rusting by coating their surface with rust-proof paints.
- Rusting can also be prevented by the application of oil or grease over iron objects. Oil and grease will prevent the iron from coming into contact with air containing moisture.
Question 158.
What are alloys? How are they obtained?
Answer:
An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal. An alloy is obtained by first melting the primary metal and then dissolving the other elements in it in definite proportions.
Question 159.
Why are alloys made? Explain with an example.
Answer:
Alloys often have properties that are different from that of the metals they contain. This makes them more useful than the pure metals alone. Alloying is a very good method of improving the properties of a metal. We can get the desired properties by this method.
For example, iron is the most widely used metal. But it is never used in its pure state. This is because pure iron is very soft and stretches easily when hot. But, if it is mixed with a small amount of carbon (about 0.05 %), it becomes hard and strong. When iron is mixed with nickel and chromium, we get an alloy called stainless steel. This alloy is hard and does not rust.
Question 160.
How are alloys prepared?
Answer:
An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal. It is prepared by first melting the primary metal. Other constituents are dissolved in the molten primary metal in definite proportions and stirred to get a uniform mixture. The mixture is then cooled to get the desired alloy.
![]()
Question 161.
Give reasons for the following:
- Platinum, sold and silver are used to make jewellery.
- Sodium, potassium and lithium are stored under oil
- Aluminium is a highly reactive metal, vet it is used to make utensils for cooking.
- Carbonate and sulphide ores are usually converted into oxides during the process of
extraction. - Alloys of iron are more useful when compared to pure iron.
- Copper loses its brown layer gradually when exposed to air.
Answer:
1. Metals such as platinum, gold and silver are the least reactive metals in nature and are placed at the bottom of the activity series. They are not affected by air, water and even by other chemicals. These metals also have bright lustre. Therefore, they are used to make jewellery.
2. Metals such as sodium, potassium and lithium are highly reactive metals. They react with water violently producing lot of heat. The heat so produced is enough to ignite hydrogen gas evolved during the reaction.
Therefore, these metals cannot be kept in the open as air also contains moisture. In order to prevent accidents, these metals are kept under non-reactive substances such as kerosene.
3. When exposed to air, aluminium changes into aluminium oxide. It gets deposited over the surface of the metal and forms a protective coating on the surface. Due to the presence of this layer, the metal becomes non-reactive and hence can be used to make cooking utensils.
4. Metal oxides can be easily reduced to metallic form with coke (C) or any other suitable reducing agent. Therefore, carbonates and sulphides are first converted into the oxide form by the processes of calcination and roasting.
5. The alloys of iron have many properties that are different but more useful than pure iron. For example, pure iron is very soft and hence stretches easily when it is hot. However, alloys of iron are quite hard and stronger than pure metal. That is why the alloys of iron are more useful than pure metal.
6. Copper reacts with moist carbon dioxide present in air forming a layer of copper oxide which appears as greenish or bluish green colour. This is why copper loses its reddish brown colour when exposed to air.
![]()
Question 162.
Name two metals that can form hydrides with hydrogen.
Answer:
Two metals that can form stable hydrides with hydrogen are sodium and calcium.
Question 163.
Name the metals that are usually alloyed with gold to make it harder.
Answer:
Copper and silver are usually alloyed with gold to make it harder.
Fill In The Blanks
1. Most reactive metal is potassium
2. Non-metals combine with oxygen to form their respective non-metallic oxides
3. Rust is nothing but hydrated oxide of iron
4. Copper is usually refined by the process of electrolysis
5. The gas displaced by metals from dilute acids is hydrogen
6. In electrolytic refining, impure metal is made as anode
7. The metal other than iron present in steel is chromium
8. The nature of metallic oxides is usually basic
9. A metal that exists in liquid state at room temperature is mercury
10. A non-metal that conducts electricity is carbon/graphite
11. Aqua regia is a mixture consisting of one volume of nitric acid and three volumes of hydrochloric acid
12. Compounds formed by the transfer of electrons from a metal to a non-metal are known as ionic compounds
![]()
Multiple Choice Questions
Question 1.
Which of the following pairs will give displacement reactions?
(A) NaCl solution and copper metal
(B) MgCl2 solution and aluminium metal
(C) FeSO4 solution and silver metal
(D) AgNO3 solution and copper metal.
Answer:
(D) AgNO3 solution and copper metal.
Question 2.
Which of the following methods is most suitable for preventing an iron article from rusting?
(A) Applying grease
(B) Applying paint
(C) Applying a coating of zinc
(D) All of the above.
Answer:
(C) Applying a coating of zinc
Question 3.
Generally non-metals are not lustrous. Which of the following non-metals is lustrous?
(A) Iodine
(B) Nitrogen
(C) Oxygen
(D) Sulphur
Answer:
(A) Iodine
![]()
Question 4.
A non-metal that exists in liquid state at room temperature is
(A) Carbon
(B) Neon
(C) Bromine
(D) Iodine
Answer:
(C) Bromine
Question 5.
An element A is soft and can be cut with a knife. This is very reactive to air and cannot be kept in the open. It reacts vigorously with water. The element is most likely to be
(A) Magnesium
(B) Sodium
(C) Phosphorus
(D) Chromium
Answer:
(B) Sodium
Question 6.
An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(A) calcium
(B) carbon
(C) silicon
(D) iron.
Answer:
(A) calcium
Question 7.
The ability of metals to be drawn into thin wires is known as
(A) Conductivity
(B) Ductility
(C) Malleability
(D) Sonority
Answer:
(B) Ductility
![]()
Question 8.
Which of the following is not true for metals?
(A) Generally metals are malleable.
(B) Metals are good conductors of heat.
(C) Metals usually give negative ions.
(D) Metals displace hydrogen gas from dilute acids.
Answer:
(C) Metals usually give negative ions.
Question 9.
Which one of the following metals would be displaced from the solution of its salts by the other three metals?
(A) Mg
(B) Ag
(C) Zn
(D) Cu
Answer:
(B) Ag
Question 10.
A metal that forms amphoteric oxide with oxygen is
(A) Aluminium
(B) Iron
(C) Magnesium
(D) Calcium
Answer:
(A) Aluminium
Question 11.
An incorrect statement with regard to magnesium metal is
(A) It bums in oxygen with a dazzling white flame.
(B) It reacts with cold water to form magnesium oxide and evolves hydrogen gas.
(C) It reacts with hot water to form magnesium hydroxide and evolves hydrogen gas.
(D) It reacts with steam to form magnesium hydroxide and evolves hydrogen gas.
Answer:
(B) It reacts with cold water to form magnesium oxide and evolves hydrogen gas.
![]()
Question 12.
During electrolytic refining of zinc, pure metal
(A) gets deposited on cathode
(B) gets deposited on anode
(C) is deposited on both cathode and anode
(D) remains in the solution
Answer:
(A) gets deposited on cathode
Question 13.
A copper vessel kept in open air slowly loses its shining brown surface and gains a green coating. It is due to the formation of
(A) CuSO4
(B) CuCO3
(C) Cu(NO3)2
(D) CuO
Answer:
(B) CuCO3
Question 14.
Which of these metals are obtained by electrolysis of their molten chlorides: sodium, calcium, iron and copper?
(A) iron and copper
(B) sodium and calcium
(C) sodium and copper
(D) calcium and copper
Answer:
(B) sodium and calcium
Question 15.
The metal stored in kerosene is
(A) Sodium
(B) Magnesium
(C) Copper
(D) Zinc
Answer:
(A) Sodium
![]()
Question 16.
Reaction between X and Y yields a compound Z. X loses an electron and Y gains an electron. Which of the following properties is not shown by Z?
(A) Conducts electricity in molten state
(B) Has high melting point
(C) Has low melting point
(D) Occurs as solid
Answer:
(C) Has low melting point
Question 17.
Food cans are coated with tin and not with zinc because
(A) zinc has a higher melting point than tin.
(B) zinc is costlier than tin.
(C) zinc is more reactive than tin.
(D) zinc is less reactive than tin.
Answer:
(C) zinc is more reactive than tin.
Question 18.
Which is the correct order as per the reactivity of the metals?
(A) Zn > Fe > Cu > Ag
(B) Fe > Zn > Cu > Ag
(C) Cu > Zn > Fe > Ag
(D) Zn > Cu > Fe > Ag
Answer:
(A) Zn > Fe > Cu > Ag
Question 19.
Aluminium is used for making cooking utensils. Which of the following properties of aluminium enables its use in making cooking utensils?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Malleability
(iv) Electro positivity
(A) (i) and (ii)
(B) (i) and (iii)
(C) (ii) and (iii)
(D) (i) and (iv)
Answer:
(B) (i) and (iii)
![]()
Question 20.
During galvanisation, iron metal is given a thin coating of
(A) chromium
(B) tin
(C) zinc
(D) copper
Answer:
(C) zinc
Question 21.
Observe the following chemical equations and identify the correct statement:
- CuSO4 + Fe → FeSO4 + Cu
- 2 AgNO3 + Cu → Cu(NO4)2 + 2 Ag
(A) Copper is more reactive than iron and silver
(B) Iron is less reactive than copper and silver
(C) Copper is more reactive than silver but less reactive than iron
(D) Silver is more reactive than copper and iron.
Answer:
(C) Copper is more reactive than silver but less reactive than iron
Match The Following
Question 1.
| Column A | Column B |
| 1. Gold | a. Can dissolve gold in it |
| 2. Bromine | b. A lustrous non-metal |
| 3. Iodine | c. A non-metal that conducts electricity |
| 4. Graphite | d. A metal that exists in liquid state at room temperature |
| 5. Aqua regia | e. A non-metal that exists in liquid state at room temperature |
| 6. Mercury | f. A gaseous metal |
| g. Metal that is least reactive |
Answer:
1 – g, 2 – e, 3 – b, 4 – c, 5 – a, 6 – d.
![]()
Question 2.
| Column A | Column B |
| 1. Gold | a. Hacmatite |
| 2. Ore of iron | b. An alloy of mercury |
| 3. Coke | c. Found in native state |
| 4. Ore of mercury | d. Copper glance |
| 5. Brass | e. Cinnabar |
| 6. Amalgam | f. Reducing agent |
| g. Alloy of copper and zinc |
Answer:
1 – c, 2 – a, 3 – f, 4 – e, 5 – g, 6 – b.