Students can Download Karnataka SSLC Science Model Question Paper 5 (Old Pattern) with Answers, Karnataka SSLC Science Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka State Syllabus SSLC Science Model Question Paper 1 (Old Pattern)
Time: 3 Hrs.
Max. Marks : 80
I. Four alternatives are provided for each question. Choose the most appropriate alternative and write it with its alphabet. 10 × 1 = 10
Question 1.
The SI unit of electric current is
(A) Ohm
(B) Volt
(C) Ampere
(D) Watt
Question 2.
The substance that is oxidised in the following chemical reaction is
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
(A) HCl
(B) MnO2
(C) MnCl2
(D) H2O
Question 3.
Red coloured light is used in traffic signals to indicate the vehicles to stop, because compared to other colours red light
(A) has high frequency
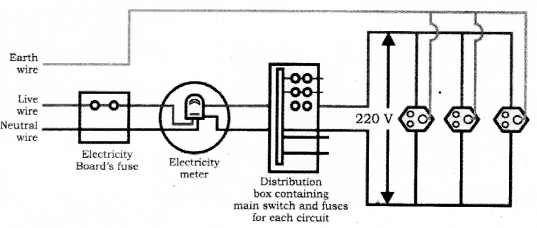
(B) scatters more
(C) has less wavelength
(D) scatters less
![]()
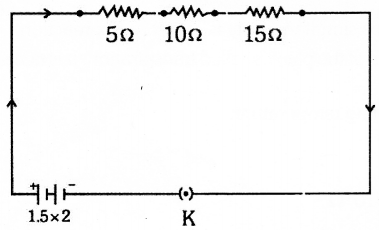
Question 4.
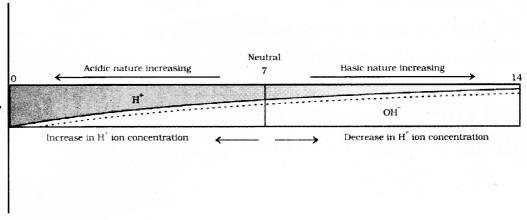
Identify the correct pair of analogous organs among the following
(A) The forelimb of man and the forelimb of a frog
(B) The wing of a butterfly and the wing of a bat
(C) The wing of a bird and the wing of a bat
(D) The forelimb of lizard and the forelimb of a frog
Question 5.
Observe the following chemical equations and identify the correct statement.
(i) \(\mathrm{CuSO}_{4}+\mathrm{Fe} \rightarrow \mathrm{FeSO}_{4}+\mathrm{Cu}\)
(ii) \(2 \mathrm{AgNO}_{3}+\mathrm{Cu} \rightarrow \mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}+2 \mathrm{Ag}\)
(A) Copper is more reactive than Iron and Silver
(B) Iron is less reactive than Copper and Silver
(C) Copper is more reactive than Silver but less reactive than Iron
(D) Silver is more reactive than Copper and Iron
Question 6.
The characterstics of the image of an object formed on the retina by the lens of the eye is
(A) Real and inverted
(B) Virtual and erect
(C) Real and erect
(D) Virtual and inverted
Question 7.
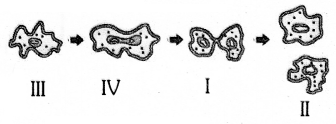
The correct order of binary fission in Leishmania is
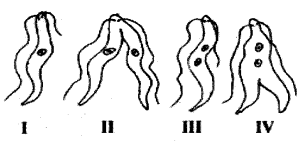
(A) II, III, IV, I
(B) I, III, IV, II
(C) IV, I, III, II’
(D) III, I, II, IV
Question 8.
The PH values of four solutions R Q, R and S are 7.8, 1.0, 13.0 and 1.4 respectively. The solution having highest hydrogen ion concentration among them is
(A) P
(B)Q
(C)R
(D) S
Question 9.
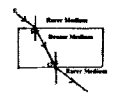
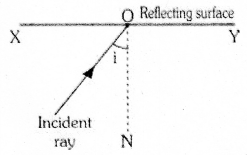
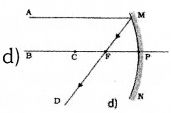
Observe the figure. The correct figure indicating the direction of the light ray FG after refraction is
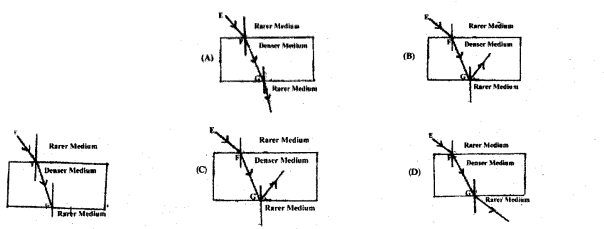
![]()
Question 10.
The watershed management
(A) increases droughts and floods
(B) increases production and income of the watershed community
(C) decreases the biodiversity of the downstrean reservoirs
(D) increases deforestation
Question 11.
Functions of certain structures of nervous system in animals are given in column ‘A’ and the names of these structures are given in column ‘B’ Match them 4 × 1 = 4
| Column – ‘A’ | Column – ‘B’ |
| i. Carries involuntary quick responses | a) Peripheral nervous system |
| ii. Controls voluntary and conscious thinking | b) Medulla |
| iii. Maintains precision in voluntary actions and balance of the body | c) Reflex Arc |
| iv. Facilitates the communication between central nervous system and the other body parts | d) Dendrite |
| e) Axon | |
| f) Cerebellum | |
| (g) Fore brain |
Answer the following questions. 7 × 1 = 7
Question 12.
The object distance of a lens is -30 cm and image distance is -10cm. Find the magnification of the lens. With the help of this, decide whether the size of the image is smaller or bigger than the size of the object.
Question 13.
What are fossils?
Question 14.
In a bakery, baking powder was not added while preparing cake. The cake obtained was hard and small in size. What is the reason for this?
Question 15.
What is geotropism?
Question 16.
Water mixed with the milk is taken in beaker A’ and sugar solution is taken in beaker ‘B’. Light is passed through both the beakers. In which beaker the path of light is visible? Why?
Question 17.
What is a chemical combination reaction?
Question 18.
In mammals and birds oxygenated blood and deoxygenated blood gets separated. Why?
Answer the following questions: 16 × 2 = 32
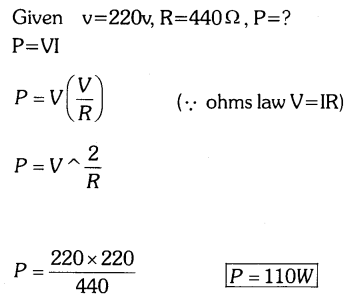
Question 19.
A potential difference of 220V is applied across a resistance of 440Ω in an electrical appliance. Calculate the current drawn and the heat energy produced in 20 seconds.
Question 20.
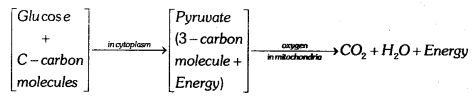
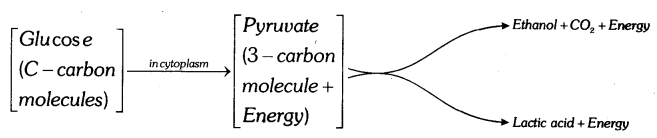
Explain the breakdown of glucose in aerobic respiration and anaerobic respiration.
OR
Explain the process of transportation of substances in phloem.
Question 21.
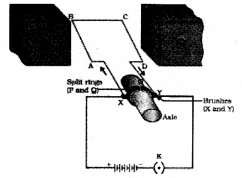
Draw the digagram of an electric motor and label the following parte
- Split rings
- Armature
Question 22.
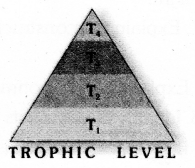
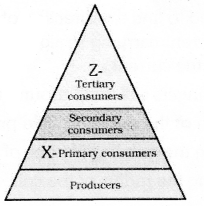
Observe the figure and answer the given questions.
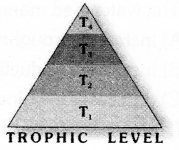
- Which trophic level has maximum number of organisms ? Why ?
- In which trophic level chemicals like DDT are accumulated in highest concentration? Why?
![]()
Question 23.
What is Myopia? Name the lens used to correct Myopia.
Question 24.
What is the resistance of a conductor? Mention the factors on which the resistance of a conductor depend.
OR
Mention the disadvantages of connecting electrical appliances in series in domestic wiring.
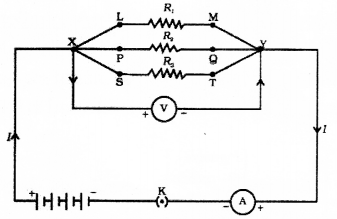
Question 25.
Draw the diagram of the electric circuit in which the resistors R1 R2 & R3 are connected in parallel including ammeter and voltmeter and mark the direction of current.
Question 26.
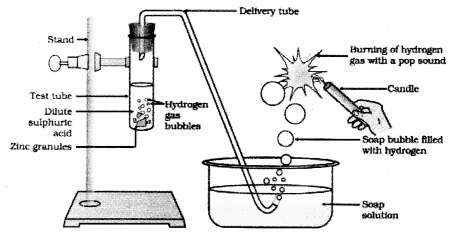
Draw the diagram of the arrangement of apparatus to know the reaction of Zinc granules with dilute sulphuric acid and testing hydrogen gas and label the part that contain zinc granules and sulphuric acid.
Question 27.
Explain the preparation of plaster of paris with the help of balanced chemical equation.
Question 28.
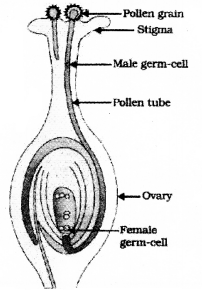
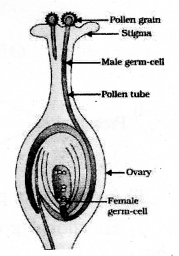
Draw the diagram showing the germination of pollen on stigma and label the following parts.
- Stigma
- Pollen Tube
Question 29.
What is placenta? Write two functions of placenta.
Question 30.
Write the four properties of ionic compounds.
OR
Write any four physical properties of metals.
Question 31.
Write the balanced chemical equations for the following chemical reactions.
- Potassium bromide reacts with Barium iodide
- Zinc carbonate is heated
OR
Which coloured fumes are obtained when lead nitrate is heated? Write the balanced chemical equation for this reaction. Name the type of this chemical reaction.
Question 32.
“Practice of reuse and recycle of materials will contribute to maintain sustainity of the environment”. Support this statement with reasons.
Question 33.
What are saturated hydrocarbons and unsaturated hydrocarbons ? Write the structure of the simplest hydrocarbon.
OR
Name the functional group in the following compounds and write their molecular formula.
- Ethanol
- Ethanoic acid
Question 34.
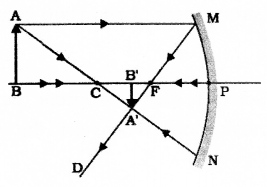
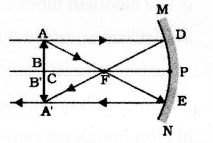
Draw the ray diagram showing the formation of image when the object is kept beyond centre of curvature
(C) of a concave mirror.
Answer the following questions. 5 × 3 = 15
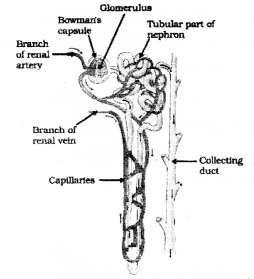
Question 35.
Draw the diagram showing the structure of a nephron and label the following parts
- Glomerulus
- Bowman’s capsule
![]()
Question 36.
State the laws of refraction. What is the meaning of “the refractive index of crown glass is 1.52”?
OR
Define the power of a lens. What is the meaning of “The power of a lens is 1 diaptor” If the power a of a lens is -2.0 D, then what type of lens is that? When an object is kept at infinity from this type of lens, what is the size of the image formed?
Question 37.
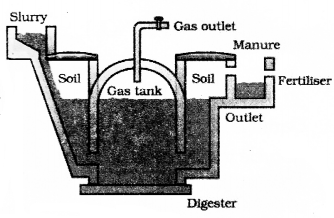
Explain the structure of a bio gas plant and the process of production of fuel in bio gas plant.
OR
“We cannot establish nuclear power reactors everywhere though large amount of electricity is produced by nuclear energy” Why? Explain.
Question 38.
The atomic numbers of two elements A and B are 11 and 12 respectively. Which element exhibits highest metallic property? Why? Write the molecular formula of the compounds formed when these elements combine with the element ‘Z’ having atomic number 8.
Question 39.
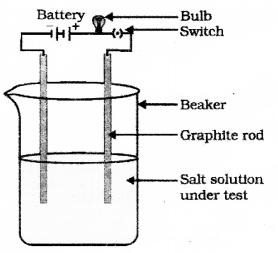
Draw the diagram of the apparatus used to test the conductivity of sodium chloride solution and label the graphite rod and the part where sodium chloride solution is present.
Answer the following questions: 3 × 4=12
Question 40.
(a) Explain the process of sex determination in human beings
(b) Why are the small number of surviving tigers a cause of worry from the point of view of genetics?
OR
(a) Traits acquired during the life time of an individual are not inherited. Why?
(b) How do Mendel’s experiments show that the traits are inherited independently? Explain.
Question 41.
(a) Explain substitution reaction with an example and chemical equation.
(b) Explain the cleansing action of soap.
Question 42.
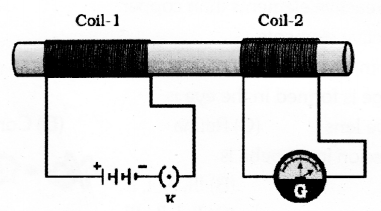
In the figure as the current changes in coil-1 the galvanometer connected to coil-2 shows deflection. Explain the phenomenon that causes this effect. Name and state the law used to know the direction of current in the device that works due to this phenomenon.
Answers
I.
Answer 1:
(C) Ampere
Answer 2.:
(A) HCl
Answer 3:
(D) Scatters less
Answer 4:
(C) The wing of bird and the wing of a bat.
Answer 5:
(A) Copper is more reactive than Iron and Silver
Answer 6:
(A) Real and Inverted
Answer 7:
(B) I, III, IV, II
Answer 8:
(B) Q
![]()
Answer 9:
(C)
Answer 10:
(B) increases production and income of the watershed community.
Answer 11:
(i) c) Reflex arc,
(ii) f) cerebellum,
(iii) g) Fore brain,
(iv) a) peripheral nervous system.
Answer 12:

Since the value is positive, image is bigger than the object.
Answer 13:
Fossils are the dead and decayed remains of organisms from the geological past i.e. preserved traces of living organisms that lived millions of years ago.
Answer 14:
Lack of accumulation of carbon dioxide gas in between the cake layers is the reason for the hardness and small size of the cake.
Answer 15:
The movement of plant part in response to gravity is called geotropic movement and the phenomenon is called geotropism.
Answer 16:
The path of light is visible in beaker A’ because, the particles present in milk scatters the light passed it.
Answer 17:
A type of chemical reaction in which a single product is formed from two or more reactants is known as chemical combination reaction.
Answer 18:
Separation of blood allows a highly efficient of supply of oxygen to the body Mammals & birds demand more energy to maintain their body temperature.
Answer 19:
Answer 20:
Breakdown of glucose in aerobic respiration glucose is converted into a 3 carbon molecule called pyruvate, which further breakdown in the presence of oxygen to form carbon dioxide and water energy is released in this process.
Breakdown of Glucose in anaerobic respiration: Clucose is converted into pyruvate. Ethyl alcohol or lactic acid is produced by the breakdown of pyruvate
Materials like sucrose is tranferred into phloem tissue using energy from ATR This increases the osmotic pressure of the tissue causing water to move into it. This pressure moves the material in the phloem to tissues which have less pressure. This allows the phloem to move material according to plants needs. For example, in the spring, sugar stored in root or stem tissue would be transported to the buds which need energy to grove.
![]()
Answer 21:
Answer 22:
- Trophic level 1 has maximum number of organisms because it includes producers.
- DDT is accumulated at Trophic level 4. It occupies the topmost place in the given food chain.
Answer 23:
Myopia is a defect in which a person can see nearly objects clearly but cannot see distant objects distinctly. It is also called near / short sightedness.
Concave lens is used to correct mopia.
Answer 24:
It is the property of a conductor to resist the flow of charges through it. It is given by
\(R=\frac{V}{I}\) Resistance of a conductor depends on
- its length
- its area of cross section
- on the nature of its material
OR
- In series combination, if any of the components fail to work, the circuit will break and then none of the components will work.
- It is not possible to connect a bulk and a heater in series because they need different valves of current to operate properly.
Answer 25:
Answer 26:
Answer 27:
On heating gypsum (CaSO4.2H2O) at 373K, it loses water molecules and become calcium sulphate hemihydrate (CaSO4.1/2H2O). This is called plaster of paris.
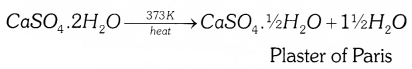
![]()
Answer 28:
Answer 29:
Placenta is a disc like structure embedded in the uterine wall. The Embryo gets nutrition from placenta.
Functions of placenta :
- It removes metabolic wastes from the embryo.
- It provides a large surface area for glucose and oxygen to pass from mother’s blood to embryo.
Answer 30:
Properties of ionic compounds:
- Ionic compounds are solids & are somewhat hard because of strong force of attraction between the positive and negative ions.
- Ionic compounds have high melting and boiling points.
- Ionic compounds are generally soluble in water & insoluble in solvents, such as kerosene, petrol etc.,
- In solution, they conduct electricity because of the presence of moving ions. Where as in the solid state they do not conduct electricity.
OR
Physical properties of metals:
- Metals are malleable and ductile.
- Most of the metals are hard. Out some metals like Li, Na, P are so soft they can be easily cut with a knife.
- Metals in their pure state have a brighter shining surface. This property is called metallic lustre.
- Most of the metals are good conductors of electricity & heat (except lead & mercury).
- Metals generally have high melting and boiling points.
Answer 31:
- \(2 K B r+B a l_{2} \rightarrow 2 K I+B a B r_{2}\)
- \(\mathrm{ZnCO}_{3} \stackrel{\text { heat }}{\longrightarrow} \mathrm{ZnO}+\mathrm{CO}_{2}\)
OR
Brown coloured fumes are obtained when lead nitrate is heated.
Balanced Chemical equation;
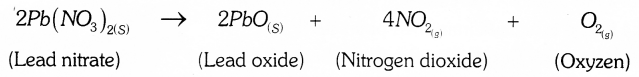
Answer 32:
Reuse means using a thing over and over again instead of throwing it away. By this we can reduce the Garbage.
Recycle involves the processing of things that are considered waste and turning them into useful products. This preserves the resources for the needs of future generations and thus contribute to maintain sustainity of the environment.
Answer 33:
The hydrocarbons in which all the carbon atoms are linked by only single bonds are called saturated hydrocarbons. The hydrocarbons in which at least one double or tripple bond is present alongwith single bonds are called unsaturated hydrocarbons.
The simplest hydrocarbon is methane
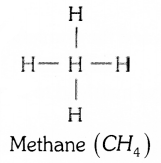
OR
| Compound | Functional group | molecular formula |
| (ii) Ethanol | Alcohol —OH | CH3CH2OH |
| (i) Ethanoic acid | Carboxylic acid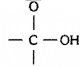 |
CH3COOH |
![]()
Answer 34:
Answer 35:
Answer 36:
Laws of refraction:
- The incident ray, the refracted ray and the normal to the interface of two transperent media at the point of incidence, all lie in the same plane.
- The ratio of the sine of the angle of incidence to the sine of angle of refraction is a constant, for the light of given colour and for the given pair of media. This law is known as snell’s law of refraction.
The refractive index of crown glass is 1.52. This means that the ratio of the speed of the light in air and the speed of light in crown glass is equal to 1.52.
OR
The power of a lens is defined as the reciprocal of its focal length it is represented by the letter R
1. Dioptre is the power of a lens whose focal length is 1 metre 1D = IM-1. If the power of a law is -2.0D. then the type of lens is concave. The size of the image is highly diminished point sized.
Answer 37:
The plant has a dome like structure built with bricks. A slum,’ of cowdung and water is made in the mixing tank from where it is fed into the digester. The digester is a sealed chanter in which there is no oxygen. Anaerobic Micro organisms that do not require oxygen decompose or break down complex compounds of the cow-dung slurry. It take a few days for the decomposition process to be complete and generate gases like methane, carbon dioxide, hydrogen and hydrogen sulphide. The bio-gas is stored in the gas tank above the digester from which they are drawn through pipes for use.
OR
Because,
- Storage and disposal of spent / used fuels and the uranium continuously decaying into harmful subatomic particles.
- Improper nuclear waste storage & disposal may lead to environmental contamination.
- There is also a rise of nuclear accidents causing due to leakage of nuclear radiation.
Answer 38:
A – 11 – Sodium
B – 12 – Magnesium
Sodium exhibits highest metallic property. Because metallic character decreases across a period.
Z- 8 – oxygen
(When sodium combines with oxygen sodium oxide is formed when magnesium combines with oxygen magensium oxide is formed). The molecular formula of the compounds formed are Na2O & 2MgO
Answer 39:
![]()
Answer 40:
(a) All human chromosomes are not paired. Most human chromosomes have a meternal and a paternal copy & We have such 22 pairs. But one pair called the sex chromosomes, is odd in not always being a perfect pair of sex chromosomes called X but men have a mismatched pair in which one is a normal sized X while the other is a short one called Y. So women are xx while men are XY. All children will inherit an X chromosome from their mother regardless of whether they are boys or girls. Thus, A child who inherits an X chromosome from her father will be a girl and one who inherits a Y chromosomes from him will be a boy.
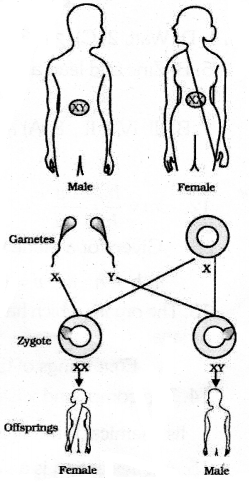
(b) Small number of surviving tigers a cause of worry from the point of view of genetics. If there is no exchange of genes between members of the species. New set of genes will not form. Diverse forms of life occur on Earth due to the evolutionary process is old tigers dies, there is no new species is present. Thus making the organisms of that species extinct.
OR
(a) Because traits aquired during the life time cannot be passed to their offsprings and are not controlled genetically. They are also called aquired traits.
Ex.: If we breed a group of mice & remove the tails of these mice by surgery the pregnency would not be tailtess, because removal of the tail cannot change the genes of germ cells of the mice and cannot direct the evolution.
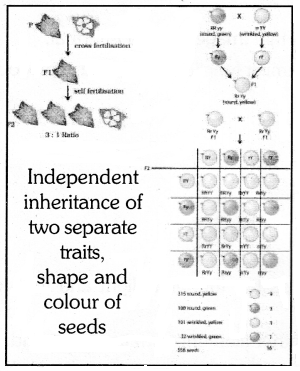
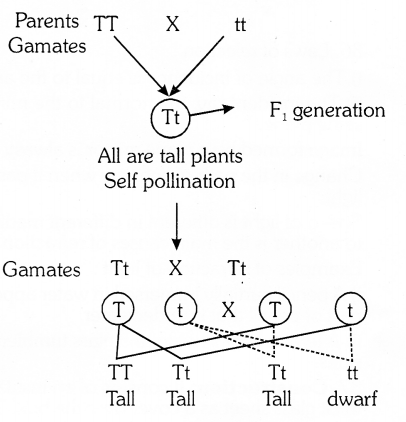
(b) Mendel used a dihydrid cross between pure pea plants to show that traits are inherited cross between pure pea plants to show that traits are inherited independently. He selected a pea plant with round yellow (RRYY) & wrinkled green (rryy). The obtained phenotypic ratio in F2 generation was 9 : 3 : 3 : 1.
This shows that the two characteristics ‘K’ & ‘Y’ are not linked to each other, so they are independently inherited.
Answer 41:
(a) A reaction in which a reagent replaces an atom or a group of atoms from the reactant is known as substitution reaction.
Eg.: In the presence of sunlight, chlorine is added to hydrocarbons at a rapid rate, here chlorine replaces hydrogen atom one by one.
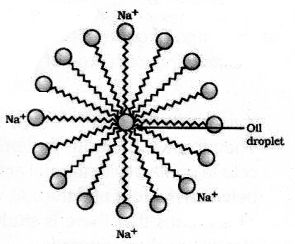
(b) The molecules of soaps are sodium or potassium salts of long chain carboxylic acids. The ionic end of soaps interacts with water while the carbon chain interacts with oil. The soap molecules thus form structures called micelles where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in pulling out the dirt in water.
![]()
Answer 42:
Electromagnetic Induction is the phenomenon that causes this effect. An electric current produced in a closed circuit by a magnetic field is called an induced current. This phenomenon is called electromagnetic induction. The law used to know the direction of current in the device is called flemmings Right Hand Rule.
Flemings right hand rule states that Stretch the thumb, forefinger and middle finger of right hand so that they are perpendicular to each other, if the forefinger indicates the direction of the magnetic field and the thumb shows he direction of motion of the conductor then the middle finger will show the direction of induced current.
Karnataka State Syllabus SSLC Science Model Question Paper 2 (Old Pattern)
I. Four alternatives are provided for each question. Choose the most appropriate alternative and write it with its alphabet. 10 × 1 = 10
Question 1.
The SI unit of electric power is
(A) Ohm
(B) Volt
(C) Ampere
(D)Watt
Question 2.
The substance that is reduced in the following chemical reaction is ZnO + C → Zn + CO
(A) C
(B) ZnO
(C)Zn
(D) CO
Question 3.
The phenomenon responsible for twinkling of stars is
(A) Atmospheric refraction
(B) Scattering of light
(C) Dispersion
(D) None of the above
Question 4.
Identify the correct pair of homologous organs among the following
(A) The forelimb of lizard and the forelimb of a frog.
(B) The wing of a bird and the wing of a bat
(C) The wing of a butterfly and the wing of bat
(D) The vermiform appendix and tail bone.
Question 5.
Observe the following chemical equations and identify the correct statement.
(i) \({ Zn }_{ 5 }+{ CuSO }_{ 4_{ (aq) } }\rightarrow { ZnSO }_{ { 4 }_{ (aq) } }+{ Cu }_{ (s) }\)
(ii) \({ Pb }_{ (s) }+{ CuCl }_{ { 2 }_{ (aq) } }\rightarrow { PbCl }_{ { 2 }_{ (aq) } }+{ Cu }_{ (s) }\)
(A) Zinc and Lead are less reactive elements than copper.
(B) Zinc and Lead are more reactive elements than copper.
(C) Zinc and Lead are unreactive elements
(D) Copper displaces zinc from zinc sulphate solution
Question 6.
The screen on which an image is formed in the eye is
(A) pupil
(B) Eye lens
(C) Retina
(D) Cornea
Question 7.
The correct order of binary fission in amoeba is
(A) IV, III, I, II
(B) III, IV,
(C) III, IV, II, I
(D) II, I, W
![]()
Question 8.
If the PH value of a solution is less than 7, then the solution is likely to be
(A) Acedic
(B) Basic
(C) Neutral
(D) Amphoteric
Question 9.
Observe the fig. the correct figure indicating the direction of the light ray AO after reflection is
Question 10.
The obstruction of forest leads to
A) Loss of forest produce.
(B) Adverse effect on quality of soil.
(C) Adverse effect on ecosystem
(D) All of the above.
Question 11.
Functions of certain hormones are given in column A’ and the names of these harmones are given in
column ‘B’ Match them.
| Column – A | Column – B |
| (i) Development of moustache & beard in males | a) Vasopressin |
| (ii) Controlling the uterus changes in menstrual cycle | b) Insulin |
| (iii) Regulates blood glucose level | c) Prejesterone |
| (iv) maintaining water and electrolyte balance | d)Testosterone |
| e) Adrenaline | |
| f) growth harmone | |
| g) Auxin |
II. Answer the following questions: 7 × 1 = 7
Question 12.
The magnification produced by plane a mirror is +1. What does it mean ?
Question 13.
What are Analogous organs ?
Question 14.
A chemical compound ‘x’ is used in glass and soap industry. Identify the compound and give its chemical formula.
Question 15.
What is reflex action ?
Question 16.
What would have been the colour of the sky, if the earth had no atmosphere & Why ?
Question 17.
What do you mean by displacement reaction ?
Question 18.
Why the walls of ventricles are thicker than the walls of atria ?
III. Answer the following questions : 16 × 2=32
Question 19.
The potential difference between two terminals of an electric iron is 220 V and the current flowing through its element is 5A. Calculate the resistance and wattage of the electric iron.
![]()
Question 20.
How are lungs (alveoli) designed in human beings to maximise the area for exchange of gases ?
OR
Explain the process of transportation of water in xylem.
Question 21.
Draw the diagram of an electric generator and label the following parts.
- Rings
- Brushes.
Question 22.
Observe the figure and answer the given questions.
- At which trophic level is maximum energy available ? Why ?
- Mention the animals which belongs to second trophic level. What are they called ?
Question 23.
What is Hypermetropia ? Name the lens used to correct hypermetropia
![]()
Question 24.
What is electric potential ? State and explain ohm’s law.
OR
Mention the advantages of connecting electrical devices in parallel with the battery instead of connecting them in series.
Question 25.
Draw the diagram of the electric circuit in which the resistors R1 R2 & R3 are connected in series including ammeter and voltmeter and mark the direction of current.
Question 26.
Draw the diagram of the arrangement of apparatus to know the electrical conductivity through an electrolyte and label the part that contain Iron nail and dilute HCl solution.
Question 27.
Explain the preperation of Bleeching powder with the help of a balanced chemical equation.
Question 28.
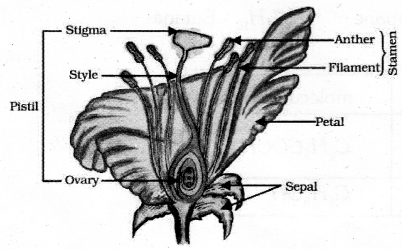
Draw the diagram of a longitudinal section of flower and label the following parts.
- Pistil
- Anther.
Question 29.
What do you mean by Regeneration ? Describe briefly how regeneration is carried out in Hydra.
Question 30.
Write a note on prevention of corrosion.
OR
Write any 4 physical properties of non-metals.
Question 31.
Write the balanced chemical equations for the following chemical reactions.
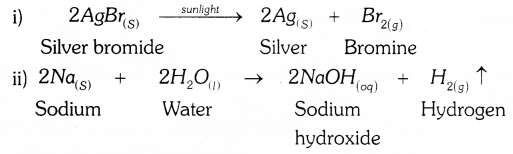
- Silver bromide on exposure to sunlight decomposes into silver and bromine.
- Sodium metal reacts with water to form sodium hydroxide and hydrogen gas.
OR
Name the products formed when the ferrous sulphate crytals are heated. Write the balanced chemical equation for this reaction. Name the type of this chemical reaction.
Question 32.
Resuse is better than recycling of materials. Give reason to satisfy this statement.
Question 33.
What is a homologous series ? Explain with an example.
OR
Name the functional group in the following compounds and write their molecular formula,
- Propanoic Acid
- Ethanol
Question 34.
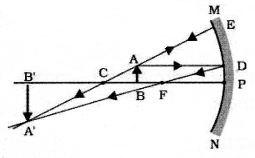
Draw the ray diagram showing the formation of image when the object is kept between centre of curvature (C) and focus (F).
IV. Answer the following questions: 5 × 3 = 15
Question 35.
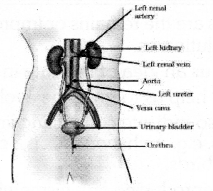
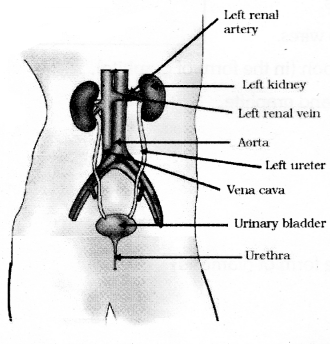
Draw a neat diagram of the human excretory system and label the following parts.
- Kidney
- Urinary bladdar.
Question 36.
State the laws of reflection. What is the nature of image formed by a plane mirror ?
OR
Define refraction of light. What is the cause of refraction ? Give two everyday examples of refraction of light.
![]()
Question 37.
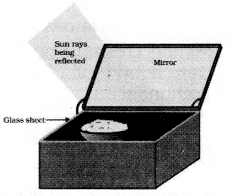
Explain the construction and working of a solar cooker.
OR
Explain the enivronmental consequences of the increasing demand for energy.
Question 38.
Identify the elements with the following property and arrange them in increasing order of their reactivity.
- An element which is soft and reactive metal.
- The metal which is an important constituent of limestone.
- The metal which exhists as liquid at room temperature.
Question 39.
Draw the diagram of apparatus used to test the action of steam on a metal and label the delivery tube and the part where the metal sample is kept.
V. Answer the following questions : 3 × 4=12
Question 40.
(a) Explain the importance of fossils in deciding evolutionary relationships ?
(b) Which type of organism will have more variations – sexually or asexually reproducing organisms ? Justify.
OR
(a) Distinguish betwen aquired trait and inherited trait with an example each.
(b) How do Mendels experiment, show the law of segregation ? Explain.
Question 41.
(a) What happens when unsaturated hydrocarbons undergo addition reaction ? Explain with a suitable reaction.
(b) Explain esterification reaction with an equation.
![]()
Question 42.
Two circular coils P & Q are kept close to each other, of which coil P carries a current. If coil P is moved towards Q, then will some current be induced in coil Q ? Give reasons for your answer and name the phenomenon involved. What happens, if coil P is moved away from Q ? Mention a method of inducing current in a coil.
Anwers
I.
Answer 1:
(D) Watt
Answer 2:
(C) Zn
Answer 3:
(A) Atmospheric refraction
Answer 4:
(A) The forelimb of lizard and the forelimb of a frog
Answer 5:
(B) Zinc and lead are more reactive elements than copper
Answer 6:
(C) Retina.
Answer 7:
(B) III, IV, I, II.
Answer 8:
(A) Acidic
Answer 9:
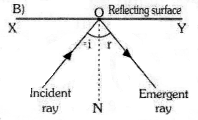
![]()
Answer 10.:
(D) All the above
Answer 11.:
i-d, ii-c, iii-b, iv-a
Answer 12:
\(m=\frac{h^{\prime}}{h}=-\frac{v}{u}\)
m = 1 indicates that the size of the image is same as that of the object.
Given for a plane mirror m = +1
∴ h1 = h & v = -u ⇒ positive sign indicates that image formed is virtual.
Answer 13:
The organs which have different origin and basic structure but show similar functions are called analogous organs.
Eg.: Wings of birds and wings of bat.
Answer 14:
The compound x is sodium carbonate.
Its chemical formula is Na2CO3
Answer 15:
A reflex action is an automatic and rapid response to a stimulus.
Eg.: Caughing, Sneezing etc.,
Answer 16:
Black, because it is due to the paricles of the atmosphere, that scatter the white light and the sky appears blue.
Answer 17:
It is a type of chemical reaction in which an element displaces another element from its compound.
Eg: Zn displaces Cu from CuSO4 solution
Equation : Zn + CuSO4 → ZnSO4 +Cu
Answer 18:
Ventricles have to pump blood into various organs with high pressure, so they have thicken walls than atria.
II.
Answer 19:
Given V=220 V, I = 5A
∴ resistance, \(A=\frac{V}{I}=\frac{220}{5} \Omega=44 \Omega\)
W.K.T. Power = V × I
= 220 × 5 = 1100 W.
![]()
Answer 20:
In lungs, balloon like structures called alveoli are present to provide maximum surface areas for the exchange of gases. The alveoli have very thin walls and contain an extensive network of blood vessels to fecilitate the exchange of gases.
OR
Xylem tissue of plants have interconnected network of vessels and trachiods of the roots stems and leaves. They form continuous system of water conducting channels reaching all parts of the plants. At the roots, cells in contact with the soil actively take up ions. This creates a difference in the concentration of these ions between the root and the soil, water, therefore, moves into the roots from the soil to eliminate the difference. This means that there is study movement of water into root system, creating a column of water that is steadily pushed upwards.
Answer 21:
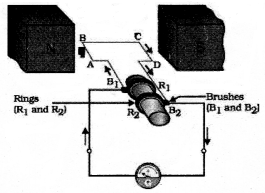
Answer 22:
- The maximum energy available atTr Because at each trophic level, a large portion of energy is utilised for the maintainance of organisms at that trophic level and some is lost as heat.
- Rabbit and Deer. They are called as primary consumers.
Answer 23:
It is a condition in which a person can see distant objects clearly but cannot see nearly objects distinctly. It is also known as far-sightedness or long sightedness.
This defect can be corrected by using a convex lens of appropriate power.
Answer 24:
Electric potential is defined as the amount of workdone when a unit positive charge is moved from infinity to a point in an electric field.
Ohms law states that the electric current flowing through a conductor is directly proportional to the potential difference applied accross its ends, provided its temperature remains the same.
OR
Advantages :
- In parallel circuit, if one electrical appliance stops working due to some defect, there all other appliances keep working normally.
- In parallel circuits each electrical appliance gets the same voltage as that of the power supply line.
- In the parallel connection of electrical appliances, the overall resistance of the household circuit is reduced due to which the current from the power supply is high.
Answer 25:
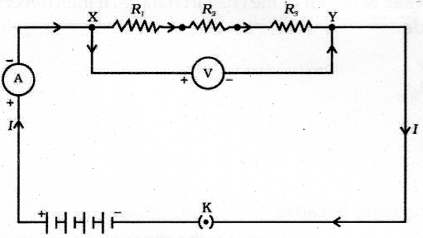
![]()
Answer 26:
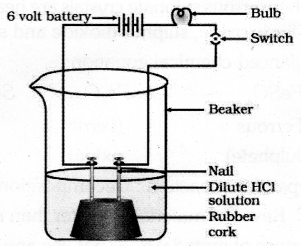
Answer 27:
Bleaching powder is produced by the action of chlorine on dry slaked lime (Ca(OH)2). It is represented as CaOCl2.
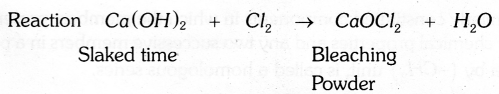
Answer 28:
Answer 29:
Regeneration is the ability of some organism to give rise to new organisms when the individual is cut or broken up into many pieces. It is seen in Hydra & planaria.
- It is carried out by specialised cells.
- When hydra is cut, these specialised cells proliferate & make large number of cells.
- From this mass of cells different cells undergo changes to become various cell types and tissues.
- There are by making each piece to grow into a separate individual.
Answer 30:
Corrosion is the process of eating away of metals by the reaction of atmospheric air and moisture. It can be prevented by.
- Galvanisation : The process of coating iron with a layer of zinc.
- Alloying: Mixing two or more metals.
- Painting, protects iron from rusting.
- Greasing or oiling: Prevents the contact the moisture & Iron.
OR
- Malleability and ductility : Non metals are neither malleable nor ductile (except diamond)
- Non – metals are brittle in nature.
Eg. : Sulphur. If it is hammared, it breaks into pieces. - Non metals do not have lustrue i.e. shining surface.
- Non metals are generally poor conductors of heat and electricity (except graphite)
Answer 31:
OR
When ferrous sulphate crystals are heated, it loses water & colour of the crystal changes. It then decomposes to ferric oxide, sulphur dioxide and sulphur trioxide.
Balanced chemical equation:
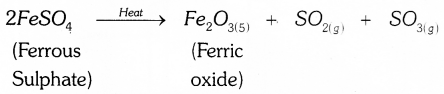
Type of the reaction: Decomposition reaction.
Answer 32:
Reuse of materials is better than recycling of materials because.
- reuse of material does not use any energy.
- it reduces the stress on environment,
- Things are maximally utilised, as they are used again and again instead of being throughing away.
![]()
Answer 33:
A series of similarly constituted components in which the members present have the same functional group and similar chemical properties and any two successive members in a particular series differ in their molecular formula by (-CH2) unit, is called a homologous series.
Ex.: alkane series CnH2n + 2
CH4 – Methane C2H4 – Ethane C3H8 – Propane C4H10 – Butane
OR
|
Compound |
functional group |
molecular formula |
|
(i) propanoic acid |
Carboxylic acid |
C2H5COOH |
|
(ii) Ethanol |
Alcohol —OH |
C2H5OH |
Answer 34:
Answer 35:
Answer 36:
Laws of relection :
- The angle of incidence is equal to the angle of relection.
- The incident ray, the normal to the mirror at the point of incidence and the reflected ray, all lie in the same plane.
Image formed by a plane mirror is always virtual and erect.
OR
Change in the path of light ray when it passes from one medium to another medium is called refraction of
light.
Speed of light is different in different media. The speed of light changes when it passes from one medium to another is the main causes of refraction.
Examples of refraction of light:
- A pencil partially immersed in water appears to be bent because of the refraction of light coming from the part of pencil that is under water.
- A lemon kept in water ina glass tumbler appears to be bigger than its actual size, when viewed from the sides.
Answer 37:
Construction: It consists of an insulated metal box which is painted black from inside the box has a thick glass sheet as a cover over the box. The reflector is a plane mirror which is attached to the box.
Working : Whenever the food is required to be cooked the solare cooker is kept in the sun. It is so adjusted that its reflector receives a strong beam of light and reflect it in the solare cooker. The reflected rays pass through the glass sheet cover and gets absorbed by the black surface in the box more and more solar radiations get trapped in the box due to green effect which increase the temperature to about 100°C-140°C.
OR
The environmental consequences of the increasing demand for energy are as following.
- The combusion of fossil fuels is producing acid rain and damaging plants, soil & aquatic life.
- The burning of fossil fuels is increasing the amount of greenhouse gas, C02 in the atmosphere.
- The cutting down of trees from forest for obtaining fire-wood is causing soil erosion and destroying wild life.
- The construction of hydro power plants is disturbing ecological balance.
- Nuclear power plants are increasing radioactivity in the environment.
Answer 38:
- Sodium is soft and reactive metal.
- Calcium
- Mercury
So, the increasing order of their reactivity is
Hg < Ca < Na or Mercury < Calcium < Sodium
Answer 39:
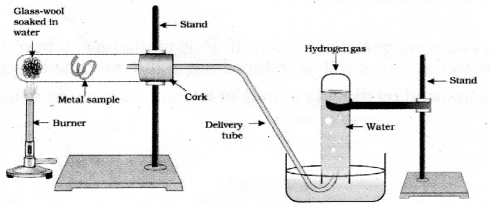
![]()
Answer 40:
a) Fossils are the remains or impressions of organisms that lived in the remote part. They are helpful in
study of evolution as.
- They give us an idea of the time in history when different species were formed or become extinct.
- They also help us to trace the evolutionary history of some animals.
- Fossils also indicate connecting link between two groups of organisms, e.g. Archaeopteryx is a connecting link between reptiles and birds.
(b) Sexually reproducing organisms will show more variations as genetic material is exchanged between homologous pair of chromosomes during cross over.
However during asexual reproduction DNA replication is the only means of variation and errors during DNA replication are not very common and frequent which may lead to variation.
OR
The characteristics developed during the lifetime of an individual, that cannot be passed on to its projeny are called aquired traits.
Eg.: If we breed a group of mice and remove the tails of thses mice by surgery, the projeny would not be tailless because removal of the tail cannot direct the evolution.
Inherited traits are those characteristics which are recieved by offsprings from their parents i.e., form one generation to other. They are controlled by genes.
Eg.: Colour of hair and eyes.
(b) Law of segregation states that ‘during gamate formation, the factor or allele of a pair segregate from each other. This is known as the law of segregation.
Mendel crossed a pure tall pea plant (TT) with pure dwarf pea plant (H) and observed that all the projeny were hybrid tall (Tt). Only one of the traits was able to express itself in the fi-generation, which is the domunant trait.
Phenotypic ratio : 3 : 1
Genotypic ratio : 1 : 2 : 1
Answer 41:
Addition of hydrogen in the presence of catalysts like pellodium or nickel to unsaturated hydrocarbons yields saturated hydrocarbons.
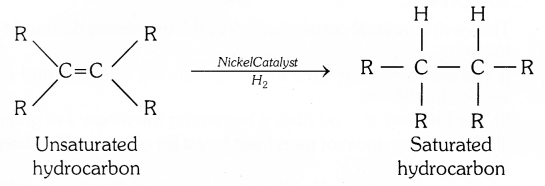
(b) Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give ester – This reaction is called esterification.
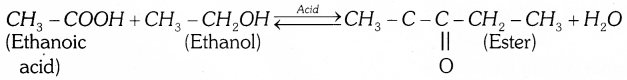
Answer 42:
When coil P is moved towards Q then current will be induced in coil Q. This is because on moving P the magnetic field associated with Q increases and so
(a) Current is induced
- The phenomenon involved is electromagnetic induction. If ‘P’ is moved away from Q, then the field associated with a will decrease and a current will be induced but in the opposite direction.
- Electric current in a coil can be induced by moving a magnet towards or away from the coil.
Karnataka State Syllabus SSLC Science Model Question Paper 3 (Old Pattern)
I. Four alternatives are provided for each question. Choose the most appropriate alternative and write it with its alphabet. 10 × 1 = 10
Question 1.
The commercial unit of electrical energy is
(a) Kilowatt Hour
(b) Kilogram
(c) Watt
(d) Volt
Question 2.
Which of the statements about the reaction below are incorrect ?
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
(a) Lead is getting reduced
(b) Carbon dioxide is getting oxidised
(c) Carbon is getting oxidised
(d) Lead oxide is getting reduced.
![]()
Question 3.
The colour of light which bends least while passing out from the prism is
(a) Bluce
(b) Red
(c) Voilet
(d) Indigo
Question 4.
In Evolutionary terms, we have more in common with a
(a) Chinese School Boy
(b) Chimpanzee
(c) Spider
(d) Baterium
Question 5.
Identify the oxidising agent in the following reaction.
CuSO4+ Zn → Cu + ZnSO4
(a) Cu
(b) ZnSO4
(c) CuSO4
(d) Zn
Question 6.
The part of the eye that controle the amount of light entering the eye is
(a) pupil
(b) Iris
(c) Cornea
(d) Retina
Question 7.
A sexual reproduction takes place through multiple fission in
(a) Amoeba
(b) Planaria
(c) Hydra
(d) Plasmodium
Question 8.
Which one of the following type of medicines is used for treating acidity.
(a) Antibiotic
(b) Antacid
(c) Analgestic
(d) Antiseptic
Question 9.
Which one of the following ray diagrams is correct for the ray of light incident on a concave mirror as shown in the figure below ?
Question 10.
Which of the following Dam is built on Ganga ?
(a) Bhakra Nangal Dam
(b) Sardar Sarovar
(c) Tehri Dam
(d) Krishna Sagar
![]()
Question 11.
Functions of plant harmones are given in column A’ and the name of the harmones are given in column ‘B’ match them
| Column – A | Column – B |
| (i) Promotes growth of the stem | a) Abscisic acid |
| (ii) Helps in vegetative growth | b) cytokinin |
| (iii) Promotes cell division | c) Gibberllin |
| (iv) Inhabits growth & Induces witting of leaves | d) Auxin |
| (e) Ethylene | |
| f) Adrenaline | |
| g) Oestrogen |
II. Answer the following questions: 7 × 1 = 7
Question 12.
Find the focal lengths of a lens of power – Z.O D, What type of lens is this ?
Question 13.
What is the basis of evolution ?
Question 14.
During summer, a milk man usually adds a very small amount of baking soda to fresh milk. Give reason.
Question 15.
What is chemotropism ?
Question 16.
What is the colour of the clear sky during day time ? Give reason for it.
![]()
Question 17.
What is redox reaction ?
Question 18.
What do you mean by transpiration ? Why it is necessary ?
III. Answer the following questions : 16 × 2 = 32
Question 19.
When 12 V battery is connected across an unknown resisto, there is a current of 2.5 mts in the circuit. Find the value of the resistance of the resistor.
Question 20.
Write the differences between aerobic respiration and anaerobic respiation.
OR
Write the advantages of transpiration in plants.
Question 21.
Draw a schematic diagram of one of the common domestic circuits.
Question 22.
Observe the figure and answer the given questions.
- Identify the primary consumer in the food web.
- If all the foxes are killed due to a disease, what will your observations about food web be ?
Question 23.
What is presbyopia, Name the lens used to correct presbyopia.
Question 24.
What is meant by electric current ? Name the components of an electric circuit.
OR
Give two advantages of heating effect of electric current.
Question 25.
Draw a closed circuit diagram consisting of a 0.5 m long nichrome wire XY, an ammeter, a voltmeter, four cells of 1.5 V each and a plug key.
Question 26.
Show the diagramatic representation of variation of PH with the change in concentration of H(+aq) and
\(O H_{(a q)}^{-}\) ions.
Question 27.
Write the possible isomers of Butane and Pentane.
Question 28.
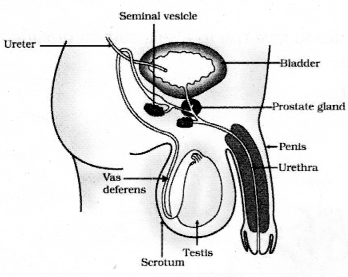
Draw the diagram showing human male reproductive system and label the following parts
- Testis
- Prostate gland.
Question 29.
What is manstruation 7 What happens when the egg is not fertilised ?
Question 30.
Differentiate between metal and non-metal on the basis of their physical properties.
OR
Explain the extraction of metals of low reactivity in the activity series.
Question 31.
Write the balanced chemical equations for the following chemical reactions.
- Reaction when glucose is oxidised / respiration.
- Formation of water from hydrogen and oxygen.
OR
A shiny brown coloured element ‘x’ on heating in air becomes black in colour. Name the element Y and the black coloured compound formed. Write the equation.
Question 32.
The damage to the ozone layer is a cause for concern, Justify the statement.
![]()
Question 33.
What is catanation ? Explain with examples.
OR
Explain the reaction of acetic acid with carbonates and bicarbonates with suitable reactions.
Question 34.
Draw the ray diagram showing the formation of image when the object is kept at focus (F1) of a convex mirror.
IV. Answer the following questions : 5 × 3 = 15
Question 35.
Draw the diagram showing the structure of heat and label the following parts.
- Left atrium
- Aorta.
- Pulmonary arteries
- Pulmonary veins.
Question 36.
Define refractive index the refractive index of diamond is 2.42. What is the meaning of this statement.
OR
Define lens, write,nature, position and relative size of image formed by concave lens.
Question 37.
Briefly explain the working of a turbines generator.
OR
List any three hazards of nuclear waste how does the disposal of nuclear waste pose a problem for the plant and animal life?
Question 38.
The atomic number of Nitrogen (N), Oxygen (O) and flourine (F) are 7, 8 & 9 respectively.
- What is the number of valence electrons in N & F?
- Name the element having smallest and largest atomic radii of any of the above three elements. Give reason for your answer.
![]()
Question 39.
Draw the diagram of the apparatus used to test the reaction of metals with salt solutions and label the
copper sulphate solution and Iron sulphate solution.
V. Answer the following questions : 3 × 4 = 12
Question 40.
a) What is evolutionary significance of the fossil Archaeopteryx ?
b) What is speciation ? List four factors that could lead to speciation.
OR
a) Name any 4 vegetables generated from a common ancestor through artificial selection rather than natural selection.
b) Why does mendel selected garden pea plant for his experiments ?
Question 41.
a) What is combustion reaction ? Explain with a suitable reaction,
b) Draw the electron dot structures for
- Ethanoic acid
- CO2
- F2
- CH4
Question 42.
The magnetic field lines due to a current through a straight conuctor is shown in the figure. Describe the process. State the rule used to find the direction of this magnetic field. How can we increase the strength of magnetic field produced by a current carrying circular coil ?
Answers
I.
Answer 1:
(a) Kilowatt hour
Answer 2:
(c) CuSO4
Answer 3:
(b) Red
Answer 4:
(a) chinees School boy,
Answer 5:
(b) Carbon dioxide is getting oxidised
![]()
Answer 6:
(b) Iris
Answer 7:
(d) Plasmodium
Answer 8:
(b) antacid
Answer 9:
Answer 10:
(c) Tehri Dam
Answer 11:
i. Auxin
ii. Gibberllin
iii. Cytokinin
iv. Abscisic Acid.
II.
Answer 12:
Given, Power = -2.0
The given lens is concave (power of concave lens is – ve)
\(\begin{array}{l}{P=\frac{1}{f(\text { in metre })}} \\ {-2.0=\frac{1}{f}} \\ {\therefore f=\frac{1}{-2} m=-50 \mathrm{cm}}\end{array}\)
∴ the focal length is = -50 cm.
Answer 13:
The changes in DNA during reproduction is the basis of evolution
Answer 14:
Baking soda does not allow milk to change to lactic acid which makes milk soor.
Answer 15:
The growth of the plant in response to a chemical stimulus and the phenomenon involved is called chemotropism.
Answer 16:
Sky appears blue during day time. The small sized particles of air scatters blue light (shorter Wavelength) that enters our eyes.
![]()
Answer 17:
The reaction in which oxidation and reduction take place simultaneously is called redox reaction.
Answer 18:
The loss of water in the form of vapour from the areal parts of the plant is called transpiration. It is necessary because.
- It creates transpiration pull.
- helps in regulating temperature.
III
Answer 19:
V = 12V, I = 25 mA = 25 × 10-3A, R = ?
\(\therefore R=\frac{V}{I}[\mathrm{By} \text { Ohm’s law }] \quad=\frac{12}{2.5 \times 10^{-3}}=4.8 \times 10^{3} \Omega\)
∴ The value of the resistance of the resistor is 4.8 × 103 Ω.
Answer 20:
| aerobic respiration | anaerobic respiration |
| 1. Takes place in presence of osygen. | i) Takes place in absence of oxygen. |
| 2. Its end products are CO2 and H2O | ii) Its end products are ethanol and carbon dioxide |
| 3. More energy is released | iii) less energy is released |
| 4. Complete oxidation of glucose takes place | iv) incomplete oxidation of glucose takes place. |
OR
Advantages of transpiration in plants
- It helps in the absorption and upward movement of water and minerals creating a transpirational poll.
- It helps in the regulation of temperature.
- It maintains a constant supply of ions to the leaves.
- It removes excess water.
Answer 21:
![]()
Answer 22:
- The primary consumers are rabbits and mice.
- If all the foxes are killed there will be no direct predator of rabbits and mice. Hence the number of rabbits and mice increase in the given ecosystem which will disturb its balance.
Answer 23:
It is a defect found in old age people. One cannot read comfortably and clearly. It arises due to the weakness of ciliary muscles & hardening of eye lens. This defect can be corrected by using bifocal or varifocal lenses which consists of both convex and concave lenses.
Answer 24:
Electric current is defined as the rate of flow of electric charge through any cross-section of a conductor in unit time.
Components of an electric circuit includes a source of current, a load, a switch / key and a fuse and connecting wire.
OR
H = I2RT Where, H is heat, t is time, R is resistance, I is current.
The above equation is known as Joules law of heating.
The law implies that the heat produced in a resistor is
- Directly proportional to the square of current for a given resistance.
- Directly proportional to resistance for a given current.
- Directly proportional to the time for which the current flows through the resistor.
Answer 25:
Answer 26:
Answer 27:
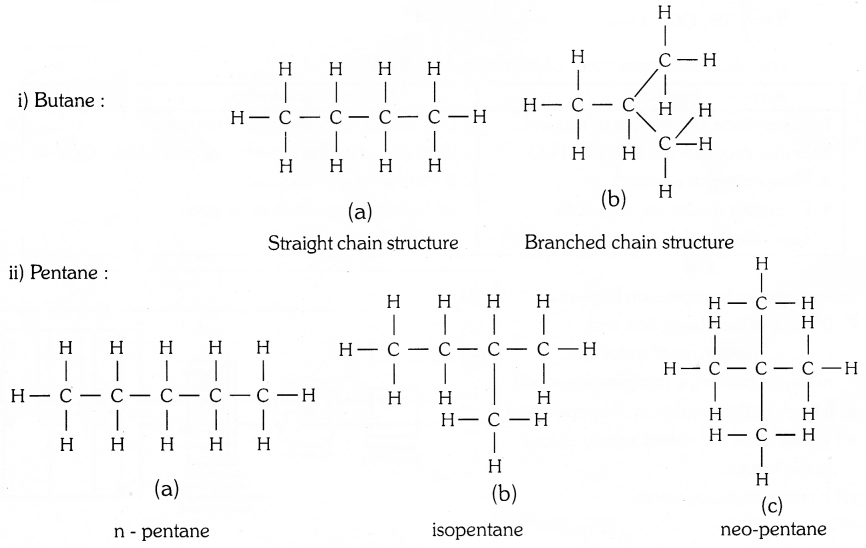
![]()
Answer 28:
Answer 29:
Menstruation is the process of breakdown and removal of the inner lining of the uterus along with the blood vessels in the form of vaginal bleeding.
When the egg is not fertilized, its starts dividing and gets implanted in the lining of the uterus.
Answer 30:
| Metals | Non – Metals |
| a. They are electropositive elements. | i. They are electronegative elements. |
| b. They show the property of malleability and ductility | ii. They do not show the property of malleability and ductility. |
| c. Metals are usually hard in nature. | iii. They are soft in nature. |
| d. They are good conductors of heat & electricity | iv. They are bad conductors of heat and electricity |
| e. They are lustrous | v. They do not have lustre. |
OR
Metals of law reactivity in the activity series are very unreactive. The oxides of these metals can be reduced to metals by heating alone.
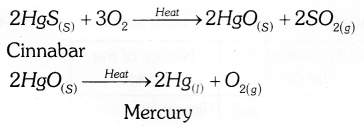
Eg. : Cinmabar (HgS) is an ore of mercury. When it is heated in air, it is first converted into mercuric oxide (HgO). Then reduced to Hg by heating.
Answer 31:
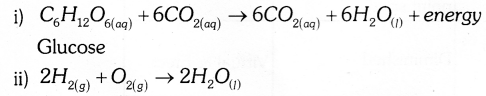
OR
Element ‘x’ is copper and the black coloured compound is copper (11) oxide
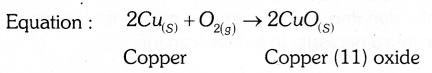
Answer 32:
Ozone layer absorbs the ultra voilet radiations. Depletion of ozone layer may lead to skin cancer in human beings, damage eyes, decrease crop yield and disturb the global rainfall pattern. Therefore the damage to the ozone layer is a cause for concern.
Answer 33:
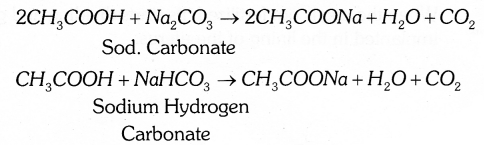
Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catanation. These compounds may have long chains of carbon or even carbon atoms arranged in rings.
Eg.: Butane, Pentane, Hexane etc,.
Ethanoic acid reacts with carbonates and hydrogen carbonates to give rise to a salt, carbon dioxide and water. The salt produced is commonly called sodium acetate.
![]()
Answer 34:
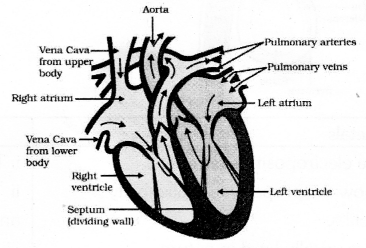
Answer 35:
Answer 36:
Refractive index is defined as the ratio of speed of light in medium 1 to the speed of light in medium 2 & it is represented as n21 and is read as refractive index of medium 2 with respect to medium 1.
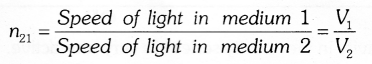
The refractive index of diamond is 2.42. This means that the ratio of speed of light in air and the speed of light in diamond is equal to 2.42.
OR
A lens is a transparent material bound by two surfaces, of which one or both surfaces are spherical.
|
Position of the object |
Position of the Image | Relative size of the image |
Nature of the image |
| At Infinity | At focus F1 | Highly diminished, point sized | Virtual & Erect |
| Between infinity and optical centre O of the lens | Between focus F1 & optical centre O | Diminished | Virtual & Erect |
Answer 37:
The simplest turbines have one moving part, a rotor-blade assembly. The moving fluid acts on the blades to spin them and impart energy to the rotor. Thus, we see that basically we need to move the fan, the rotor blade, with speed which would turn the shaft of the dynamo and convert the mechanical energy into electrical energy – the form of energy which has become a necessity in todays scenario.
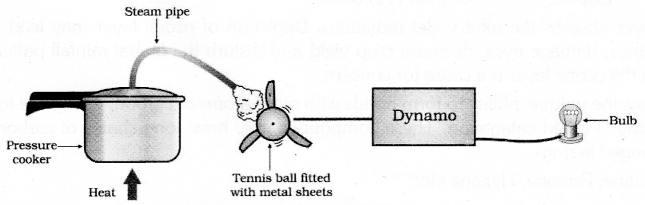
Hazzards:
- The nuclear releases harmful radiations and hence need safe disposal.
- It is highly toxic in nature.
- If not disposed properly it may contaminate the environment.
The improper disposal of nuclear waste car cause threat to plants and animals in the following ways.
If any plant or animal gets exposed to these nuclear waste or nuclear radiations the radiations can penetrate
into the cells and can cause the genetic disorder and may also lead to cancer. The plant and animals will
soon be absnormal and extinct.
![]()
Answer 38:
(i) Valence electron of N=5
Valence electron of F=7
(ii) Smallest radius = F
Largest radius = N
Reason : Atomic radius decreases from left to right across a period due to increase in the force of attraction between nucleus and valence electrons.
Answer 39:
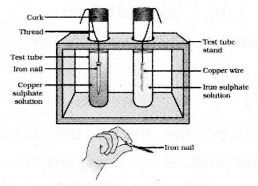
Answer 40:
(a) Archaeopteryx indicates that birds have evolved from reptiles. They are the connecting link between birds and reptiles and have characteristics of both these organisms,
(b) speciation is the development of one or more new species pre-existing species, four factors that could lead to speciation are
- Genetic drift
- Mutation
- Natural selection
- migration
OR
a)
- Cabbage selected for short distance between leaves.
- Broccoli selected for arrested flower development.
- Couliflower selected for sterile flowers forming a large flower.
- Kohlrabi selected for a swollen edible stem.
- Xale selected for large leaves.
b) Mendal selected garden pea plants for his experiments because.
- They grow quickly & easier to study.
- Pea plants can be crossed or self-pollinated and have a flower structure that limits accidental contact.
- garden pea has various contracting characters.
Answer 41:
a. All Carbon compounds burn in presence oof oxygen to give carbon dioxide and water vapours Heat and light are also retard during this process this reaction is called combusion reaction.
Eg.: C + O2 → CO2 + heat + light
CH4 + 2O2 → CO2 + 2 H2O + heat + light
b.
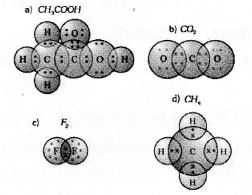
![]()
Answer 42:
The magnetic field lines around a current carrying straight conductor are concentric circles whose centres tie on the wire. The concentric circles representing the magnetic field around a current carrying straight wire become larger and larger as we move away from it. Thus the magnetic field produced by a given current in the conductor decreases as the distance from it increases.
The rule used to find the direction of this magnetic field is called right – hand thumb rule it states that if you hold the current carrying straight conductor in your right hand such that the thumb points towards the direction of the current.
Then your fingers will wrap around the conductor in the direction of the field lines of the magnetic field.
The strength of the magnetic field produced by a current carrying circular coil can be increased by
- increasing the number of turns of wire in the coil.
- Increasing current flowing through the coil,
- Decreasing the radius of the coil.
Karnataka State Syllabus SSLC Science Model Question Paper 4 (Old Pattern)
I. Four alternatives are provided for each question. Choose the most appropriate alternative and write it with its alphabet. 10 × 1 = 10
Question 1.
The device that is used to detect current in an electric circuit is
a) Voltmeter
b) Galvanometer
c) Ammeter
d) Resistor
Question 2.
Which of the following chemical equation represents double displacement reaction.
a) 2mg + O2 → 2Mgo
b) \({ CaCO }_{ 3 }\underrightarrow { heat } CaO+{ CO }_{ 2 }\)
c) CuSO4 + Zn → ZnSO4 + Cu
d) AgNO3 + NaCl → AgCl + NaNO3
Question 3.
The increasing order of the deviation of the colours observed in a spectrum through a prism is
a) Red, orange, yellow, green, blue, Indigo and violet.
b) Violet, Indigo, Blue, green, yellow, orange, red.
c) Red orange, green, blue, yellow, Indigo, violet.
d) Violet, Indigo, green, yellow, blue, orange, red.
Question 4.
An example for homologous organs is
a) Our arm and a dog’s for – leg
b) Our teeth and an elephant’s tusks,
c) Potato and runners of gross
d) all of the above
Question 5.
The metals which do’not react with water at all are
a) Lead, copper silver and gold
b) Magnesium, Iron and Zinc
c) Calcium, Potassium and Sodium
d) None of these
Question 6.
The type of the lens which is used to correct hypermetropia is
a) Concave lens
b) Convex lens
c) Both Concave and convex lenses
d) either concave or convex
Question 7.
The mode of reproduction shown in the figure is
a) Fragmentation
b) Regeneration
c) Fission
d) Budding
![]()
Question 8.
The P” Value of a solution which turns red litmus to blue is likely to be
a) 1
b) 4
c) 5
d) 14
Question 9.
No matter how far you stand from a mirror, your image appears erect. The mirror is likely to be
a) only plane
b) only concave
c) only convex
d) either plane or conve
Question 10.
An approach towards the conservation of forest is
a) Industrial activities
b) Housing and road formation projects
c) construction of big dams
d) Afforestation
Question 11.
Match the following :
| A | B |
| 1. Insulin | a. Plant harmone |
| 2. Thyroxin | b. Harmone secreted by a pancreas |
| 3. Oestrogen | c. Harmone responsible for growth and metabolism. |
| 4. Cytokinin | d. Harmone secreted by ovaries |
| e. Harmone secredted by testis | |
| f. Harmone secreted by Adrenal gland |
II. Answer the following questions 7 × 1 = 7
Question 12.
The image of a candle flame placed at a distance of 45 cm from a spherical lens is formed on a screen placed at a distance of 90 cm. from the lens identify the type of the lens and calculate the focal length.
Question 13.
Define evolution.
Question 14.
For making a cake, if at home your mother used baking soda instead of baking powder in cake then how will it affect the taste of cake and why ?
Question 15.
hat is chemotropism ?
Question 16.
A glass prism is able to produce a spectrum when white light passes through it but glass slab does not produce any spectrum, why it is so ?
Question 17.
What is a displacement reaction (or substitution reaction) ?
Question 18.
Why do lungs always have residual volume of air ?
III. Answer the following questions : 16 × 2 = 32
Question 19.
An electric motor takes 5 A from a 220 V line determine the power of the motor and the energy consumed in 2h.
Question 20.
- Name the substance that is exodised in the body during respiration
- Why are lungs divided into very small sac like structures ?
OR
Which mechanism plays an important role in transportation of water in plants ?
- During day time
- At night.
Question 21.
Draw a schematic diagram of domestic wiring and label the following parts.
Question 22.
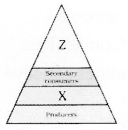
Write the appropriate names of the trophic levels Z and X in the figure given below.
Question 23.
A person is able to see objects clearly only when these are lying at distances between 50 cm and 300 cm from his eye.
![]()
Question 24.
Why are the coils of electric toasters and electric irons made of an alloy rather than a pure metal ?
OR
What is the need of using combination of resistances in electrical circuits ? Define equivalent resistance ?
Question 25.
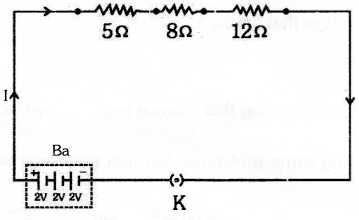
Draw a schematic diagram of a circuit consisting of a battery of three cells 2 V each, a 5Q resistor, and 8 Q resistor and a 12 Q resistor and plug key, all connected in series.
Question 26.
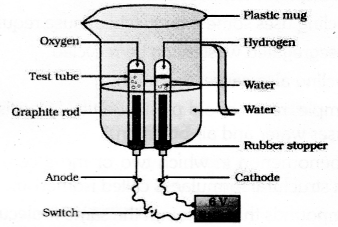
Draw a neat labelled diagram to show the electrolysis of water.
Question 27.
What is a neutralisation reaction ? Give example.
Question 28.
Draw the diagram showing the germination of pollen on stigma and label the following parts.
- pollen tube
- pollen grain
- ovary
- stigma.
Question 29.
What are fallopian tubes ? Write two functions of fallopian tubes (oviducts).
Question 30.
State any four physical properties of non-metals.
OR
a) Name the solvent in which ionic compounds are generally soluble :
b) Why are ionic compound can conduct electricity, when taken in molten form ?
Question 31.
Write the balanced chemical equations for the following chemical reactions
- Caclium hydroxide + carbon dioxide → calcium carbonate + water
- Aluminium + Copper chloride → Aluminium chloride + Copper
OR
Why does the colour of copper sulphate solution change when an iron nail is dipped in it ?
Write the balanced chemical equation for this reaction. Name the type of this chemical reaction.
![]()
Question 32.
With the help of an example show that reuse strategy is better than recycling.
Question 33.
What is isomerism ? Draw two structural isomers of butane.
OR
Name the functional group in the following compounds and write their molecular formula
- propanal
- acetone
Question 34.
Draw the ray diagram showing the formation of image when the object is kept at the centre of curvature
(C) of a concave mirror.
Question 35.
Draw the diagram showing the structure of a excretory system in human beings and label the following
parts.
- Aorta
- Ureter
Question 36.
State mirror formula. Is the same formula applicable to both concave and convex mirrors ? Define magnification for a spherical mirror.
OR
Define the principal focus of a concave mirror the radius of curvature of a spherical mirror is 20 cm. What is its focal length ? Name the mirror that can give an erect and enlarged image of an object.
Question 37.
What is the principle of working of solar cooker ? Write two major drawbacks of all solar heating devices.
OR
How is nuclear energy generated ? State the main advantages and hazards of nuclear energy
Question 38.
The elements \(_{4} B e,_{12} \mathrm{Mg} \text { and }_{20} \mathrm{Ca}\) each having two valence electrons in their valence shells are in periods 2, 3 and 4 respectively of the modern periodic table. Answer the following questions.
a) In which group should they be ?
b) Which one of these is least reactive ?
c) Which one of them has the largest atomic size ?
![]()
Question 39.
Draw the diagram of the apparatus used to test the conductivity of sodium chloride solution and label its parts.
IV. Answer the following questions:
Question 40.
What is meant by speciation ? What factors could lead to the rise of new species ?
OR
a) Differentiate between dominant and recessive traits.
b) ‘Genes control traits’ explain this statement with an example.
Question 41.
a) Explain combination reaction with an example and chemical equation,
b) Explain.the terms with example in each case : i) oxidation & ii) Reduction.
Question 42.
The following figure indicates the device that converts electrical energy to mechanical energy.
Answer the following questions based on this figure.
- Name the device given in the figure.
- Fleming’s which rule, is applied to find the direction of force in its conductivity.
- What is the principle of this device ?
- What type of rings it has and what is the role of these rings in the device ?
Answers
I
Answer 1:
b) Galvanometer
Answer 2:
d) AgNO3 + NaCl → AgCl + NaNO3
Answer 3:
a) Red, orange, yellow, green, blue, Indigo and violet
Answer 4:
d) all of the above
Answer 5:
a) Lead, copper silver and gold
Answer 6:
b) Convex lens
Answer 7:
d) Budding
Answer 8:
d) 14
Answer 9:
d) either plane or convex
![]()
Answer 10:
d) Afforestation
Answer 11:
1(b), 2(c), 3(d), 4 (a)
Answer 12:
Here u=-45 cm, v= +90 cm
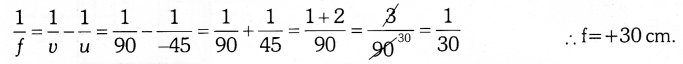
As f is positive, the lens used is convex lens with focal lenght 30 cm.
Answer 13:
The gradual unfolding events by which new organisms evolved from pre-existing organisms through changes since the beginning of life is said to be evolution.
Answer 14:
The taste of the cake becomes bitter as the medium becomes too basic due to baking soda.
[Note: Baking powder contains baking soda, corn and tartaric Acid]
Answer 15:
The movement of a plant in response to a chemical stimulus is called chemotropism
Answer 16:
In a glass slab, both the refreshing faces are parallel. All the emergent rays are parallel to the incident rays. Hence there is neither dispersion nor any deviation of light.
Answer 17:
The reaction in which an atom or a group of atoms in the molecule is displaced by another atom or a group of atoms is Called displacement or substitution reaction.
Answer 18:
The lungs always contains a residual volume of air so that there is sufficient time for oxygen to be absorbed and for the carbondioxide to be released.
Answer 19:
Here, I = 5A, V=220 V, t = 2h=7200 Sec
Power, P —VI
= 220 × 5 = 1100 W
Energy consumed = P × t
= 1100 W × 7200 S
= 7920000 J
Answer 20:
- Glucose is oxidised in the body during respiration.
- Lungs are divided into very small sac – like structures to increase the respiratory surface area. This facilitates efficient exchange of oxygen and CO2 between the air and the blood.
OR
- During day time suction pressure creates by transpiration (evaporation of water) helps in absorption and upward movement of water and minerals dissolved in it from roots to the leaves, us to the top of the plant.
- At night, the effect of root pressure [created by movement of water from the soil to the xyloem of roots] in transport of water is more important.
![]()
Answer 21:
Answer 22:
Answer 23:
a) The person is suffering from both myopia and hypermetropia.
b) The person requires bifocal lenses to increase his range of vision. The upper part of the bifocal lens is a concave lens which facilitates distant vision which facelitates near vision.
Answer 24:
This is because of two reasons.
- Alloys have higher resistivity than that of their constituent metals.
- Alloys donot oxidise [or burn] readily at high temperature.
OR
The need of using combination of resistances is to obtain a desired value of current in an electrical circuit, it can be done in three ways they are i) series combination, ii) parallel combination and iii) mixed combination.
Equivalent resistance : If a single resistance can replace the combination of resistances in such a manner that the current in the circuit remains unchanged, then that single resistance is called the equivalent resistance.
Answer 25:
Answer 26:
Answer 27:
The reaction between an acid and a base to give salt and water is called neutralization reaction.
Answer 28:
![]()
Answer 29:
These are the two tubes attached to the uterus one on either side.
- The fallopian tubes carry egg (ova) from ovary to the womb (uterus)
- Fertilisation of the egg by the sperm may take place in the fallopian tube
Answer 30:
The four physical properties of non-metals are:
- Non-metals are brittle, i.e. they cannot be beaten into sheets.
- Non-metals are non-ductile, i.e., they cannot be drawn into wires.
- They are bad conductors of heat and electricity except carbon (in the form of graphite).
- They are dull [non-lusturous] [donot shine] except iodine and graphite.
- They have comparitively low melting & boiling points.
- They have low densities.
- They may be solid, liquid or gas at room temperature.
Eg.: bromine ⇒ liquid non metal. - Most of the solid non-metals are soft except carbon (in the form of diamond)
- They are not strong i.e. they have low tensile strength.
OR
a) Water is the solvent in which ionic compounds are generally soluble.
b) Ionic compounds contain ions become mobile in molten state and conduct electricity whereas in solid form, ions are fixed in a lattice and the compound does not conduct electricity.
Answer 31:
- Ca(OH)2 + CO2 → CaCO3+H2O
- 2Al + 3CuCl2 → 2AlCl3 + 3Cu
OR
When an iron nail is dipped in copper sulphate solution, it becomes brownish in colour because iron has replaced copper from its solution.
Thus the amount of copper sulphate decreases and its colour fades.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
This is a displacement reaction.
Answer 32:
- Recycling uses some energy while reuse requires energy not to that amount.
- Only segregated wastes can be recycled.
- Recycling also causes pollution.
For example, reuse of old plastic bottles / platisis for better than recycling them which requires chemicals and causes water and air pollution.
Answer 33:
The phenomenon in which two or more compounds having same molecular formula may exist with different structural formulae is called isomerism.
The compounds that containm the same molecular formula but different structures are called isomers.
Structural isomers of butane C4H10 are
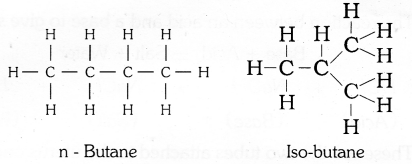
OR
(i)

ii) Acetone : [or propanone]
Functional groups : Ketone, Molecular formla : CH3COCH3
Answer 34:
Answer 35:
Answer 36:
Mirror formula : It is a mathematical relation between the object distance is image distance ‘v’ and focal length ‘f of a spherical mirrors. This relation is
\(\frac{1}{u}+\frac{1}{v}=\frac{1}{f} \text { or } \frac{1}{v}+\frac{1}{u}=\frac{1}{f}\)
This formula is applicable to all concave and convex mirrors Magnification by a spherical mirror:
The ratio of the height of the image to the height of the object is called magnification. It is denoted by in 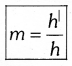 Where h| = image height, h = object height
Where h| = image height, h = object height
Note: Magnification ‘m’ is also related to the object distance (u) and image distance (v). It can be expressed as
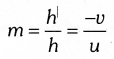
A negative sign in the value of magnification indicates that the image is real.
A positive sign in the value of magnification indicates that the image is virtual.
OR
Principle focus of a concave mirror is a point on the principal axis at which a beam of light incident parallel to the principal axis converges after reflection from the concave mirror.
Focal length of a spherical mirror = 1/2 × radius of curvature
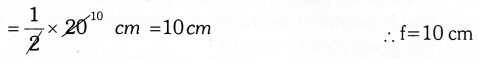
Concave mirror gives an erect and enlarged image of an object.
![]()
Answer 37:
Principle of working of solar cooker: The glass sheet placed in the box alloes the sun heat (rays) to be absorbed by the black surface of box but does not allow the heat so absorbed to leave the box easily. This is because the glass has the peculiar properly to pass through it only such infrared radiations which have wavelength near to visible light. It does not allow infrared radiations of higher waverlength to come out of the solar cooker. Thus the temperature. Now the wavelength of infrared radiations inside the box is higher than those from outside. Hence, heat from the box does not pass out through glass sheet. Further inside black of the cooker absorb maximum heat.
Major Disadvantages:
- Solar energy is available in highly diffused focus.
- Proper devices to concentrate and store solar energy are not available as yet.
OR
When a heavy nucleus of an unstable atom like uranium – 235 breaks up an bombardment with low energy neutrons into smaller atoms. It emits a, p and y radiations with large amount of energy. This process is called nuclear fission.
Advantages :
- It is used to generate electricity.
- It is used to treat diseases like cancer.
- The fuel once filled can be used for a long time.
Disadvantages: It causes health hazards due to emission of harmful radiations like a, p and y radiations,
- safe disposable of nuclear wastes is a big handicap.
Answer 38:
a) These have valency of 2 and so they should be in Group II.
b) \(_{4} B e\) is least reactive as reactivity. Increases down a group.
c) \(_{20} C a\) has largest atomic size as size increases down a group.
Answer 39:
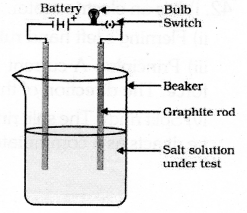
Answer 40:
Speciation: Geographical isolation of two populations of same species over several generations may result in genetic driff (random change in gene frequency) and the population which once had similar genetic make up would become different.
Further natural section acts on these isolated populations differently. If these differences accumulated over generations, the isolated, populations become so different they cannot reproduce with each other (reproductive isolation) and two new species are said to be formed This called speciation.
Factors which lead to the new species are :
- Accumulated variations favourable to the natural environment.
- Geographical isolation of a population.
- Action of different environmental factors on these isolated populations.
- Reproductive isolation and change of number of chromosomes results in the formation of new species.
OR
(a)
| Dominant Trait | Recessive trait |
| The trait which is expressed in FI generation is called dominant trait | The trait which remains hidden in presence of dominant trait is called recessive trait. |
a) Dominant Trait
Recessive trait The trait which is expressed in The trait which remains hidden in presence of
FI generation is called dominant trait dominant trait is called recessive trait,
b) Genes control trait: A gene codes for the formation of a particular protein controlling a specific characteristic of the organism.
Example: In pea, the gene T is responsible for tallness of the plant. This gone T gives instructions to the plant cell to make a lot of plant growth harmones. Due to formation of excess plant growth harmones, the plant will grow too much and thus becomes tall. Thus genes control traits.
![]()
Answer 41:
a) Combination reaction: The reaction of two or more substances (either elements or compounds) to produce a new substance is called a combination reaction
Eg. : Magnesium (Mg) on burning in air combines with oxygen to give magnesium oxide.
2Mg(s)+ O2(g) → 2MgO(s)
b)
i) Oxidation: It is a process in which
a) oxygen or any electronegative element is added up
b) hydrogen or any electropositive element is removed.
Eg.: Sulphur + Oxygen → Sulphur dioxide
S + O2 → SO2 ( Addition of oxygen to sulphur)
ii) Reduction: It is a process in which
a) hydrogen or an electropositive element is added up
b) oxygen or electronegative element is removed.
Eg.: Hydrogen reacts with chlorine to form Hydrogen chloride.
Cl2(g) + H2(g) → 2HCl(g) ( Addition of hydrogen to chlorine)
![]()
Answer 42:
- It is an electric motor.
- Fleming’s left hand rule is applied for the direction of the force on a conductor.
- Principle: A current carrying conductor when placed in a magnetic field experiences a mechanical force. The direction of this force is given by fleming’s left hand rule.
- Split rings: The split ring reverses the direction of the current in the armature coil after every half rotation, i.e. it acts as a commutator which allows the armature coil to rotate continuously on the same direction.
Karnataka State Syllabus SSLC Science Model Question Paper 5 (Old Pattern)
I. Four alternatives are provided for each question. Choose the most appropriate alternative and write it with its alphabet. 10 × 1 = 10
Question 1.
The SI unit of electric current is
(A) Ohm
(B) Volt
(C) Ampere
(D) Watt
Question 2.
The substance that is oxidised in the following chemical reaction is
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
(A) HCl
(B) MnO2
(C) MnCl2
(D) H2O
Question 3.
Red coloured light is used in traffic signals to indicate the vehicles to stop, because compared to other colours red light
(A) has high frequency
(B) scatters more
(C) has less wavelength
(D) scatters less
Question 4.
Identify the correct pair of analogous organs among the following
(A) The forelimb of man and the forelimb of a frog
(B) The wing of a butterfly and the wing of a bat
(C) The wing of a bird and the wing of a bat
(D) The forelimb of lizard and the forelimb of a frog
![]()
Question 5.
Observe the following chemical equations and identify the correct statement.
(i) \(\mathrm{CuSO}_{4}+\mathrm{Fe} \rightarrow \mathrm{FeSO}_{4}+\mathrm{Cu}\)
(ii) \(2 \mathrm{AgNO}_{3}+\mathrm{Cu} \rightarrow \mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}+2 \mathrm{Ag}\)
(A) Copper is more reactive than Iron and Silver
(B) Iron is less reactive than Copper and Silver
(C) Copper is more reactive than Silver but less reactive than Iron
(D) Silver is more reactive than Copper and Iron
Question 6.
The characterstics of the image of an object formed on the retina by the lens of the eye is
(A) Real and inverted
(B) Virtual and erect
(C) Real and erect
(D) Virtual and inverted
Question 7.
The correct order of binary fission in Leishmania is
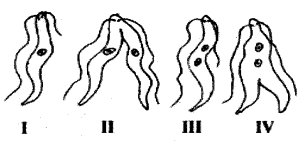
(A) II, III, IV, I
(B) I, III, IV, II
(C) IV, I, III, II’
(D) III, I, II, IV
Question 8.
The PH values of four solutions R Q, R and S are 7.8, 1.0, 13.0 and 1.4 respectively. The solution having highest hydrogen ion concentration among them is
(A) P
(B)Q
(C)R
(D) S
Question 9.
Observe the figure. The correct figure indicating the direction of the light ray FG after refraction is
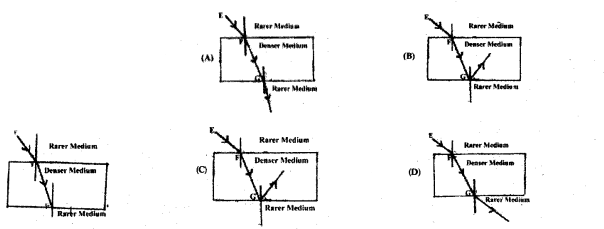
Question 10.
The watershed management
(A) increases droughts and floods
(B) increases production and income of the watershed community
(C) decreases the biodiversity of the downstrean reservoirs
(D) increases deforestation
![]()
Question 11.
Functions of certain structures of nervous system in animals are given in column ‘A’ and the names of these structures are given in column ‘B’ Match them 4 × 1 = 4
| Column – ‘A’ | Column – ‘B’ |
| i. Carries involuntary quick responses | a) Peripheral nervous system |
| ii. Controls voluntary and conscious thinking | b) Medulla |
| iii. Maintains precision in voluntary actions and balance of the body | c) Reflex Arc |
| iv. Facilitates the communication between central nervous system and the other body parts | d) Dendrite |
| e) Axon | |
| f) Cerebellum | |
| (g) Fore brain |
Answer the following questions. 7 × 1 = 7
Question 12.
The object distance of a lens is -30 cm and image distance is -10cm. Find the magnification of the lens. With the help of this, decide whether the size of the image is smaller or bigger than the size of the object.
Question 13.
What are fossils?
Question 14.
In a bakery, baking powder was not added while preparing cake. The cake obtained was hard and small in size. What is the reason for this?
Question 15.
What is geotropism?
Question 16.
Water mixed with the milk is taken in beaker A’ and sugar solution is taken in beaker ‘B’. Light is passed through both the beakers. In which beaker the path of light is visible? Why?
![]()
Question 17.
What is a chemical combination reaction?
Question 18.
In mammals and birds oxygenated blood and deoxygenated blood gets separated. Why?
Answer the following questions: 16 × 2 = 32
Question 19.
A potential difference of 220V is applied across a resistance of 440Ω in an electrical appliance. Calculate the current drawn and the heat energy produced in 20 seconds.
Question 20.
Explain the breakdown of glucose in aerobic respiration and anaerobic respiration.
OR
Explain the process of transportation of substances in phloem.
Question 21.
Draw the digagram of an electric motor and label the following parte
- Split rings
- Armature
Question 22.
Observe the figure and answer the given questions.
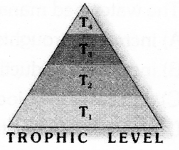
- Which trophic level has maximum number of organisms ? Why ?
- In which trophic level chemicals like DDT are accumulated in highest concentration? Why?
![]()
Question 23.
What is Myopia? Name the lens used to correct Myopia.
Question 24.
What is the resistance of a conductor? Mention the factors on which the resistance of a conductor depend.
OR
Mention the disadvantages of connecting electrical appliances in series in domestic wiring.
Question 25.
Draw the diagram of the electric circuit in which the resistors R1 R2 & R3 are connected in parallel including ammeter and voltmeter and mark the direction of current.
Question 26.
Draw the diagram of the arrangement of apparatus to know the reaction of Zinc granules with dilute sulphuric acid and testing hydrogen gas and label the part that contain zinc granules and sulphuric acid.
Question 27.
Explain the preparation of plaster of paris with the help of balanced chemical equation.
Question 28.
Draw the diagram showing the germination of pollen on stigma and label the following parts.
- Stigma
- Pollen Tube
![]()
Question 29.
What is placenta? Write two functions of placenta.
Question 30.
Write the four properties of ionic compounds.
OR
Write any four physical properties of metals.
Question 31.
Write the balanced chemical equations for the following chemical reactions.
- Potassium bromide reacts with Barium iodide
- Zinc carbonate is heated
OR
Which coloured fumes are obtained when lead nitrate is heated? Write the balanced chemical equation for this reaction. Name the type of this chemical reaction.
Question 32.
“Practice of reuse and recycle of materials will contribute to maintain sustainity of the environment”. Support this statement with reasons.
Question 33.
What are saturated hydrocarbons and unsaturated hydrocarbons ? Write the structure of the simplest hydrocarbon.
OR
Name the functional group in the following compounds and write their molecular formula.
- Ethanol
- Ethanoic acid
Question 34.
Draw the ray diagram showing the formation of image when the object is kept beyond centre of curvature
(C) of a concave mirror.
Answer the following questions. 5 × 3 = 15
Question 35.
Draw the diagram showing the structure of a nephron and label the following parts
- Glomerulus
- Bowman’s capsule
Question 36.
State the laws of refraction. What is the meaning of “the refractive index of crown glass is 1.52”?
OR
Define the power of a lens. What is the meaning of “The power of a lens is 1 diaptor” If the power a of a lens is -2.0 D, then what type of lens is that? When an object is kept at infinity from this type of lens, what is the size of the image formed?
![]()
Question 37.
Explain the structure of a bio gas plant and the process of production of fuel in bio gas plant.
OR
“We cannot establish nuclear power reactors everywhere though large amount of electricity is produced by nuclear energy” Why? Explain.
Question 38.
The atomic numbers of two elements A and B are 11 and 12 respectively. Which element exhibits highest metallic property? Why? Write the molecular formula of the compounds formed when these elements combine with the element ‘Z’ having atomic number 8.
Question 39.
Draw the diagram of the apparatus used to test the conductivity of sodium chloride solution and label the graphite rod and the part where sodium chloride solution is present.
Answer the following questions: 3 × 4=12
Question 40.
(a) Explain the process of sex determination in human beings
(b) Why are the small number of surviving tigers a cause of worry from the point of view of genetics?
OR
(a) Traits acquired during the life time of an individual are not inherited. Why?
(b) How do Mendel’s experiments show that the traits are inherited independently? Explain.
Question 41.
(a) Explain substitution reaction with an example and chemical equation.
(b) Explain the cleansing action of soap.
Question 42.
In the figure as the current changes in coil-1 the galvanometer connected to coil-2 shows deflection. Explain the phenomenon that causes this effect. Name and state the law used to know the direction of current in the device that works due to this phenomenon.
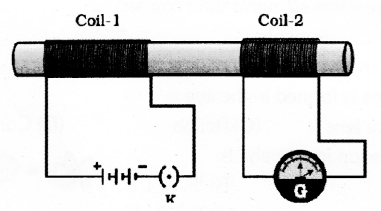
Answers
I.
Answer 1:
(C) Ampere
![]()
Answer 2.:
(A) HCl
Answer 3:
(D) Scatters less
Answer 4:
(C) The wing of bird and the wing of a bat.
Answer 5:
(A) Copper is more reactive than Iron and Silver
Answer 6:
(A) Real and Inverted
Answer 7:
(B) I, III, IV, II
Answer 8:
(B) Q
Answer 9:
(C)
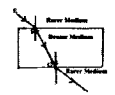
Answer 10:
(B) increases production and income of the watershed community.
Answer 11:
(i) c) Reflex arc,
(ii) f) cerebellum,
(iii) g) Fore brain,
(iv) a) peripheral nervous system.
Answer 12:

Since the value is positive, image is bigger than the object.
Answer 13:
Fossils are the dead and decayed remains of organisms from the geological past i.e. preserved traces of living organisms that lived millions of years ago.
Answer 14:
Lack of accumulation of carbon dioxide gas in between the cake layers is the reason for the hardness and small size of the cake.
Answer 15:
The movement of plant part in response to gravity is called geotropic movement and the phenomenon is called geotropism.
![]()
Answer 16:
The path of light is visible in beaker A’ because, the particles present in milk scatters the light passed it.
Answer 17:
A type of chemical reaction in which a single product is formed from two or more reactants is known as chemical combination reaction.
Answer 18:
Separation of blood allows a highly efficient of supply of oxygen to the body Mammals & birds demand more energy to maintain their body temperature.
Answer 19:
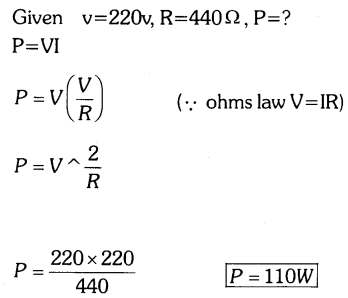
Answer 20:
Breakdown of glucose in aerobic respiration glucose is converted into a 3 carbon molecule called pyruvate, which further breakdown in the presence of oxygen to form carbon dioxide and water energy is released in this process.
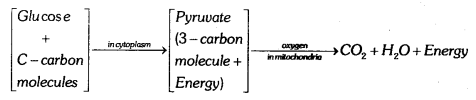
Breakdown of Glucose in anaerobic respiration: Clucose is converted into pyruvate. Ethyl alcohol or lactic acid is produced by the breakdown of pyruvate
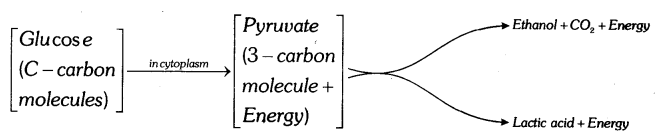
Materials like sucrose is tranferred into phloem tissue using energy from ATR This increases the osmotic pressure of the tissue causing water to move into it. This pressure moves the material in the phloem to tissues which have less pressure. This allows the phloem to move material according to plants needs. For example, in the spring, sugar stored in root or stem tissue would be transported to the buds which need energy to grove.
Answer 21:
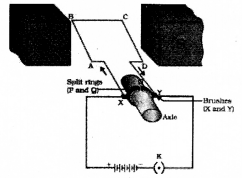
![]()
Answer 22:
- Trophic level 1 has maximum number of organisms because it includes producers.
- DDT is accumulated at Trophic level 4. It occupies the topmost place in the given food chain.
Answer 23:
Myopia is a defect in which a person can see nearly objects clearly but cannot see distant objects distinctly. It is also called near / short sightedness.
Concave lens is used to correct mopia.
Answer 24:
It is the property of a conductor to resist the flow of charges through it. It is given by
\(R=\frac{V}{I}\) Resistance of a conductor depends on
- its length
- its area of cross section
- on the nature of its material
OR
- In series combination, if any of the components fail to work, the circuit will break and then none of the components will work.
- It is not possible to connect a bulk and a heater in series because they need different valves of current to operate properly.
Answer 25:
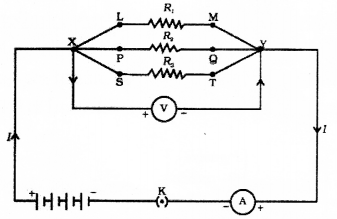
Answer 26:
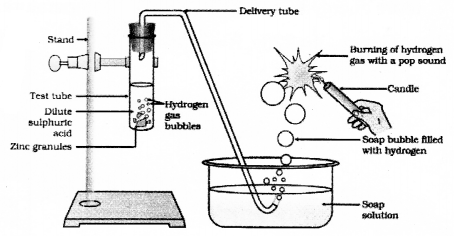
Answer 27:
On heating gypsum (CaSO4.2H2O) at 373K, it loses water molecules and become calcium sulphate hemihydrate (CaSO4.1/2H2O). This is called plaster of paris.
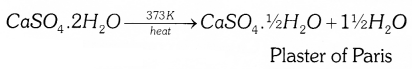
Answer 28:
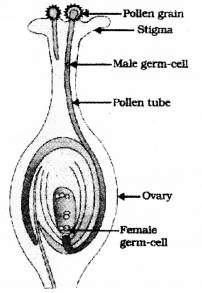
![]()
Answer 29:
Placenta is a disc like structure embedded in the uterine wall. The Embryo gets nutrition from placenta.
Functions of placenta :
- It removes metabolic wastes from the embryo.
- It provides a large surface area for glucose and oxygen to pass from mother’s blood to embryo.
Answer 30:
Properties of ionic compounds:
- Ionic compounds are solids & are somewhat hard because of strong force of attraction between the positive and negative ions.
- Ionic compounds have high melting and boiling points.
- Ionic compounds are generally soluble in water & insoluble in solvents, such as kerosene, petrol etc.,
- In solution, they conduct electricity because of the presence of moving ions. Where as in the solid state they do not conduct electricity.
OR
Physical properties of metals:
- Metals are malleable and ductile.
- Most of the metals are hard. Out some metals like Li, Na, P are so soft they can be easily cut with a knife.
- Metals in their pure state have a brighter shining surface. This property is called metallic lustre.
- Most of the metals are good conductors of electricity & heat (except lead & mercury).
- Metals generally have high melting and boiling points.
Answer 31:
- \(2 K B r+B a l_{2} \rightarrow 2 K I+B a B r_{2}\)
- \(\mathrm{ZnCO}_{3} \stackrel{\text { heat }}{\longrightarrow} \mathrm{ZnO}+\mathrm{CO}_{2}\)
OR
Brown coloured fumes are obtained when lead nitrate is heated.
Balanced Chemical equation;
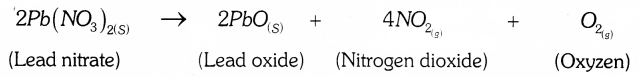
Answer 32:
Reuse means using a thing over and over again instead of throwing it away. By this we can reduce the Garbage.
Recycle involves the processing of things that are considered waste and turning them into useful products. This preserves the resources for the needs of future generations and thus contribute to maintain sustainity of the environment.
Answer 33:
The hydrocarbons in which all the carbon atoms are linked by only single bonds are called saturated hydrocarbons. The hydrocarbons in which at least one double or tripple bond is present alongwith single bonds are called unsaturated hydrocarbons.
The simplest hydrocarbon is methane
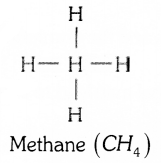
OR
| Compound | Functional group | molecular formula |
| (ii) Ethanol | Alcohol —OH | CH3CH2OH |
| (i) Ethanoic acid | Carboxylic acid |
CH3COOH |
Answer 34:
![]()
Answer 35:
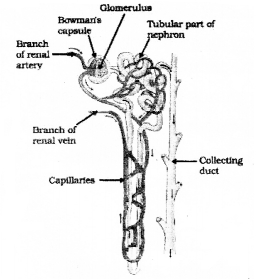
Answer 36:
Laws of refraction:
- The incident ray, the refracted ray and the normal to the interface of two transperent media at the point of incidence, all lie in the same plane.
- The ratio of the sine of the angle of incidence to the sine of angle of refraction is a constant, for the light of given colour and for the given pair of media. This law is known as snell’s law of refraction.
The refractive index of crown glass is 1.52. This means that the ratio of the speed of the light in air and the speed of light in crown glass is equal to 1.52.
OR
The power of a lens is defined as the reciprocal of its focal length it is represented by the letter R
1. Dioptre is the power of a lens whose focal length is 1 metre 1D = IM-1. If the power of a law is -2.0D. then the type of lens is concave. The size of the image is highly diminished point sized.
Answer 37:
The plant has a dome like structure built with bricks. A slum,’ of cowdung and water is made in the mixing tank from where it is fed into the digester. The digester is a sealed chanter in which there is no oxygen. Anaerobic Micro organisms that do not require oxygen decompose or break down complex compounds of the cow-dung slurry. It take a few days for the decomposition process to be complete and generate gases like methane, carbon dioxide, hydrogen and hydrogen sulphide. The bio-gas is stored in the gas tank above the digester from which they are drawn through pipes for use.
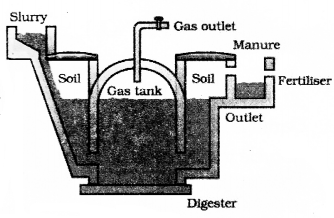
OR
Because,
- Storage and disposal of spent / used fuels and the uranium continuously decaying into harmful subatomic particles.
- Improper nuclear waste storage & disposal may lead to environmental contamination.
- There is also a rise of nuclear accidents causing due to leakage of nuclear radiation.
Answer 38:
A – 11 – Sodium
B – 12 – Magnesium
Sodium exhibits highest metallic property. Because metallic character decreases across a period.
Z- 8 – oxygen
(When sodium combines with oxygen sodium oxide is formed when magnesium combines with oxygen magensium oxide is formed). The molecular formula of the compounds formed are Na2O & 2MgO
Answer 39:
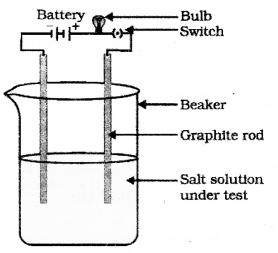
Answer 40:
(a) All human chromosomes are not paired. Most human chromosomes have a meternal and a paternal copy & We have such 22 pairs. But one pair called the sex chromosomes, is odd in not always being a perfect pair of sex chromosomes called X but men have a mismatched pair in which one is a normal sized X while the other is a short one called Y. So women are xx while men are XY. All children will inherit an X chromosome from their mother regardless of whether they are boys or girls. Thus, A child who inherits an X chromosome from her father will be a girl and one who inherits a Y chromosomes from him will be a boy.
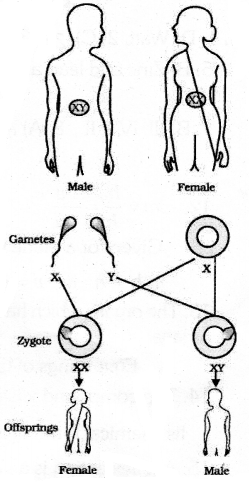
(b) Small number of surviving tigers a cause of worry from the point of view of genetics. If there is no exchange of genes between members of the species. New set of genes will not form. Diverse forms of life occur on Earth due to the evolutionary process is old tigers dies, there is no new species is present. Thus making the organisms of that species extinct.
OR
(a) Because traits aquired during the life time cannot be passed to their offsprings and are not controlled genetically. They are also called aquired traits.
Ex.: If we breed a group of mice & remove the tails of these mice by surgery the pregnency would not be tailtess, because removal of the tail cannot change the genes of germ cells of the mice and cannot direct the evolution.
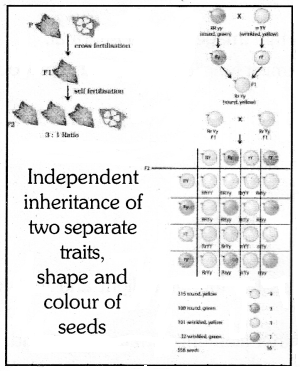
(b) Mendel used a dihydrid cross between pure pea plants to show that traits are inherited cross between pure pea plants to show that traits are inherited independently. He selected a pea plant with round yellow (RRYY) & wrinkled green (rryy). The obtained phenotypic ratio in F2 generation was 9 : 3 : 3 : 1.
This shows that the two characteristics ‘K’ & ‘Y’ are not linked to each other, so they are independently inherited.
![]()
Answer 41:
(a) A reaction in which a reagent replaces an atom or a group of atoms from the reactant is known as substitution reaction.
Eg.: In the presence of sunlight, chlorine is added to hydrocarbons at a rapid rate, here chlorine replaces hydrogen atom one by one.
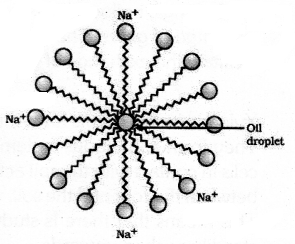
(b) The molecules of soaps are sodium or potassium salts of long chain carboxylic acids. The ionic end of soaps interacts with water while the carbon chain interacts with oil. The soap molecules thus form structures called micelles where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in pulling out the dirt in water.
![]()
Answer 42:
Electromagnetic Induction is the phenomenon that causes this effect. An electric current produced in a closed circuit by a magnetic field is called an induced current. This phenomenon is called electromagnetic induction. The law used to know the direction of current in the device is called flemmings Right Hand Rule.
Flemings right hand rule states that Stretch the thumb, forefinger and middle finger of right hand so that they are perpendicular to each other, if the forefinger indicates the direction of the magnetic field and the thumb shows he direction of motion of the conductor then the middle finger will show the direction of induced current.