Students can Download Karnataka SSLC Science Model Question Paper 1 with Answers, Karnataka SSLC Science Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka State Syllabus SSLC Science Model Question Paper 1 With Answers
Time: 3 Hours
Max Marks: 80
I. Four alternatives are provided for each of the following questions or incomplete statements. Choose the most appropriate alternative and write with its alphabet. ( 8 × 1 = 8 )
Question 1.
Which of the following groups contain only biodegradable items
A) Grass, flowers, and leather
B) Grass, wood and plastic
C) Fruit, peels, cake and lime juice
D) Cake, wood and glass
Answer:
C) Fruit, peels, cake and lime juice
Question 2.
The embryo gets nutrition from the mother’s blood with the help of a special tissue called
A) Zygote
B) Utreus only
C) Placenta
D) None of these
Answer:
C) Placenta
Question 3.
One of the main aim of conservation is to try and preserve the ______ we have inherited.
A) Ecosystem
B) Biodiversity
C) Environment
D) Universal
Answer:
B) Biodiversity
![]()
Question 4.
Which of the following terms does not represent electrical power in a circuit?
A) I2R
B) IR2
C) VI
D) V2/R
Answer:
B) IR2
Question 5.
By which reaction metal is obtained from metal oxide
A) Liquification
B) Reduction
C) Calcination
D) Roasting
Answer:
B) Reduction
Question 6.
Conversion of electrical signal to chemical signal occurs at:
A) Dendrite of a neuron
B) Axon of a neuron
C) Nerve ending of a neuron
D) Cell body of a neuron
Answer:
C) Nerve ending of a neuron
Question 7.
Which of the following reaction will never occur
A) Cu + H2SO4 → CuSO4 + H2
B) Mg + H2SO4 → MgSO4 + H2
C) 2Al + 6HCl → 2AlCl3 + 3H2
D) Fe + 2HCl → FeCl + H2
Answer:
A) Cu + H2SO4 → CuSO4 + H2
![]()
Question 8.
Sodium carbonate is a basic salt because it is a salt of
A) Strong acid and strong base
B) Weak acid and weak base
C) Strong acid and weak base
D) Weak acid and strong base
Answer:
D) Weak acid and strong base
II. Answer the following questions ( 8 × 1 = 8 )
Question 9.
What are autosomes and sex chromosomes?
Answer:
Human cell contain 23 pairs of chromosomes out of 23 pairs, 22 pairs are called autosomes, rest of 1 pair, which determine the sex of child is called sex chromosomes.
Question 10.
What does the high level of total coliform count in river Ganga indicate?
Answer:
The high level of total coliform count in river Ganga indicates that the water is contaminated by disease causing microorganisms (mainly sewage).
Question 11.
Why are we not able to see the things clearly when we come out of a dark room?
Answer:
When we are in dark, pupil size is bigger, as we come out of dark room, its size needs to become smaller. For that time interval the person is unable to see.
![]()
Question 12.
What is an electric current? Write its SI unit.
Answer:
The rate of flow of charges through a conductor is called an electric current. Its SI unit is ampere (A).
Question 13.
In a bakery, baking powder was not added while preparing cake. The cake obtained was hard and small in size. What is the reason for this.
Answer:
Baking powder is used for baking cakes. It contains sodium hydrogen carbonate, which breaks down when heated to form CO2 gas. The CO2 help to make the cakes light and fluffy.
Question 14.
Why do we consider tungsten as suitable material for making the filament of a bulb?
The filament of an electric bulb is usually made of tungsten because of its higher resistivity value (5.56 × 10 Ω m) and its high melting point (3422°C).
Question 15.
Where do plants get each of the raw materials required for photosynthesis?
Answer:
- Carbon dioxide : Air, through stomata.
- Water : Soil through roots.
- Sunlight; From the sun, present in green parts of the body absorbed by chlorophyll molecules plant body.
![]()
Question 16.
What is combustion?
Answer:
The complete oxidation of a carbon compound leading to the formation of CO2 and H2O is called combustion.
III. Answer the following questions. ( 8 × 2 = 16 )
Question 17.
“Two areas of study namely evolution and classification are interlinked” Justify the statement?
Answer:
- Different forms of organisms or life have evolved during the course of evolution. Classification deals with grouping of there organisms into groups and subgroups based on their similarities and differences.
- The characteristics commonly seen in any two species will be closely related and they will be closely related and they will have a more recent or common ancestor.
- Thus, classification helps tracing the evolutionary relationships between the two organisms. Hence it can be said that classification and evolution are interlinked.
Question 18.
Draw the diagram of a simple electric motor. Label the following parts.
i) Split rings ii) Brushes
Answer:
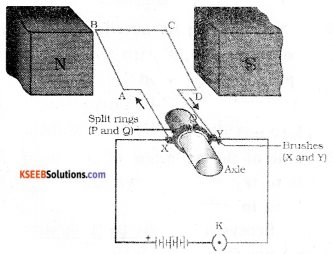
![]()
Question 19.
What is the advantages of disposable paper cups over disposable plastic cups?
Answer:
Disposable paper cups are biodegradable as they are degraded by saprophytic bacteria and fungi. The disposable plastic cups remain in the environment for longer period of time causing pollution as they are non-biodegradable.
OR
What does energy flow diagram indicate?
Answer:
Energy flow diagram indicates the following:
- Energy flow is undirectional.
- As much energy is wasted only a limited number of trophic levels (3 or 4) are found in the food chain.
- Biological magnification of harmful non – biodegradable substances occur in food chain.
Question 20.
Draw the diagram of the apparatus used in electrolytic refining of copper and label the electrode where pure copper is deposited.
Answer:
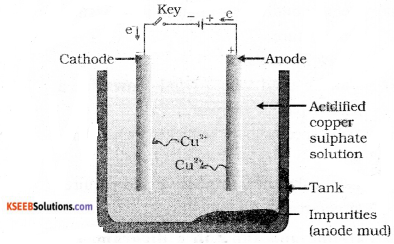
Question 21.
Why are decomposition reaction called the opposite of combination reactions? Write equations for these reactions.
Answer:
In decomposition reaction, a single compound splits to give rise to two or more simple substances. In combination reaction, two or more simpler substances combine together to form a single compound. Hence they are opposite to each other.
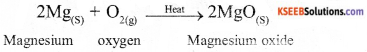
Example of decomposition reactions :
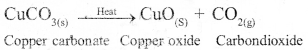
Example of combination reaction
OR
Give an example of redox reaction, naming the substances which are oxidised and reduced.
Answer:
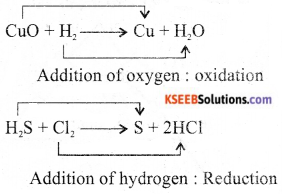
![]()
Question 22.
Draw the structure of a neuron and label and following parts.
Answer:
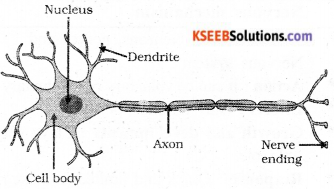
Question 23.
What is short circuit? State one condition that leads to it. Name the device in the household that acts as a safety measure for it.
Answer:
When live wire and neutral wire come in direct contact, a heavy current in the circuit is called short circuiting.
Factor : Insulation of wire is damaged.
Safety device : Electric fuse.
Question 24.
Draw the ray diagram showing the position of the object and image, to get the virtual erect image whose size is enlarged by using convex lens.
Answer:
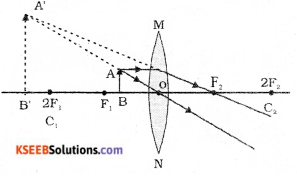
IV. Answer the following questions ( 9 × 3 = 27 )
Question 25.
a) Define Resistance of conductor.
Answer:
The property of the conductor to oppose the flow of charges through it.
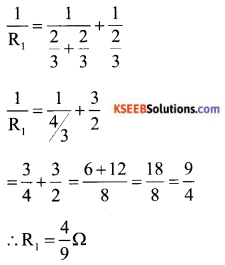
b) Calculate the equivalent resistance between A and B from the following combination of resistors.
Answer:
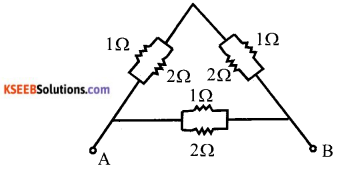
Here 1 Ω and 2 Ω are connected in parallel. So equivalent resistance
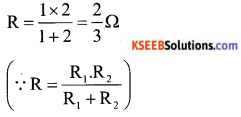
Two resistor of \(\frac{2}{3}\) Ω are in series is parallel to \(\frac{2}{3}\) Ω resistor.
Equivalent resistance between A and B
![]()
Question 26.
What is Geothermal energy? How can it be harnessed to produce electrical energy.
Answer:
The energy obtained from the earth’s crust is called Geothermal energy.
When underground water comes in contact with hot spots, steam is generated. The steam is taken out through pipe to a turbine to produce electricity.
OR
List three factors responsible for the wind. State three limitations in harnessing wind energy.
Answer:
Three factors responsible for wind:
- Uneven heating of earth’s surface.
- Rotation of the earth.
- Local condition – change in pressure, temperature.
Three limitations:
- Can be used where wind blows for a greater part of the year.
- Wind speed should be higher than 15 km / hr.
- Establishment is expensive and requires large area.
Question 27.
Define and show in a diagram, the following terms relating to concave mirrors.
i) Aperture
ii) Radius of curvature
List any four uses of concave mirror.
Answer:
i) The diameter of the reflecting surface of the mirror is called aperature.
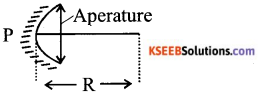
ii) The radius of the sphere of which reflecting surface of the spherical mirror forms a part is called the radius of curvature of the spherical mirror.
Uses of concave mirror are :
- Shaving mirror.
- Reflectors in automobile head light and torches.
- By dentists to see back side of tooth.
- Reflector of solar furnaces.
Question 28.
What happens when a solution of baking soda is heated? Write chemical equation for the same. Name the product which is responsible for making the bread or cake spongy and fluffy.
Answer:
When baking soda is heated it decomposes to produce sodium carbonate, water and carbondioxide gas.
![]()
CO2 gas produce during the reaction makes the cake or bread spongy and fluffy.
OR
i) Write the name given to bases that are highly soluble in water. Give an example.
ii) How is tooth decay related to pH.
iii) Why does bee – sting cause pain and irratiation?
Rubbing of baking soda on the sting area gives relief. How?
Answer:
i) Alkali, NaOH
ii) Tooth decay starts when the pH of the mouth is lower than 5.5. It can be prevented by using toothpaste which are generally basic.
iii) Bee sting has acid that cause pain and irritation.
Baking soda being alkaline, Neutralises acid and given relief.
Question 29.
How does a solenoid behave like a magnet? Can you determine the north and south poles of a current carrying solenoid with the help of a bar magnet?
Answer:
When electric current flows through a solenoid, magnetic field is set up around the solenoid. The pattern of the magnetic field is same as that of the magnetic field of a bar magnet. One end of the solenoid behaves as north pole and the other end of the solenoid behaves as south pole.
To determine the north and south poles of a current carrying solenoid with the help of a bar magnet, suspend it with a strong thread. Now bring the north pole of a bar magnet towards one end of the solenoid. If the solenoid attracts towards the magnet then the face of the solenoid is south pole. If the bar magnet moves away from the solenoid, then the face of the solenoid is the north pole.
![]()
Question 30.
a) What is meant by least distance of distinct vision?
Answer:
It is the minimum distance up to which eye can see clearly and is called the least distance of distinct vision.
b) What happens to the image distance in the eye when we increase the distance of an object from the eye.
Answer:
The size of the eye can change, so the image distance is fixed when we increase the distance if the eye does not change, due to power of accommodation of the eye, focal length of the eye lens is changed, which compensates the increase in object distance. Hence image distance remains fixed and image is formed on the retina of the eye.
OR
a) Why are we not able to see the things clearly when we come out of a dark room?
Answer:
When we are in dark, pupil size is bigger, as we come out of dark room, its size needs to become smaller. For that time interval person is unable to see.
b) Why does the sun appear reddish early in the morning.
Answer:
During sunrise, the light rays coming from the sun have to travel a greater distance in the earth’s atmosphere before reaching our eyes.
In this journey, the shorter wave length of lights are scattered out and only longer wavelength are able to reach our eyes. Since blue colour had a shorter wavelength and red colour has longer wave length, the red colour is able to reach our eyes after the atmospheric scattering of light. Therefore the sun appear reddish early in the morning.
Question 31.
a) Define speciation?
Answer:
Speciation is arising of a new species from a sub-population of a species which is geographically or reproductively isolated over a long period of time from the other population of the same species.
b) Mention the sex chromosomes present in human male and human female with the help of a flow chart determine genetically in human beings the sex of the offspring if the sperm carrying x chromosomes fertilizers the egg.
Answer:
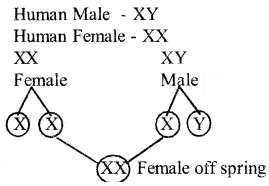
Question 32.
a) Define 1 dioptre of power of a lens.
Answer:
The SI unit of power of lens is dioptre which is denoted by the letter D. 1 Dioptre is defined as the power of a lens of focal length 1 meter.
b) The refractive index of diamond is 2.42. What is the meaning of this statement.
Answer:
The refractive index of diamond is 2.42 this means that the speed of light in diamond will reduce by a factor of 2.42 as compared to its speed in air. In other words the speed of light in diamond is 2.42 times less than the normal speed of light in vacuum.
c) In which type of lens linear magnification is always less than one?
Answer:
Concave lens always has linear magnification less than one, because it always gives diminished images.
![]()
Question 33.
a) What will be the formula and electron dot structure of cyclopentane.
Answer:
The formula for cyclopentane is C5H10. Its electron dot structure is given below.
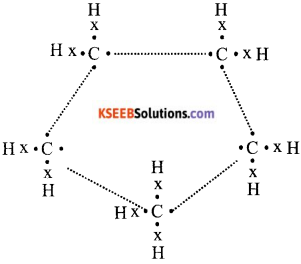
b) How would you distinguish experimentally between an alcohol and carboxylic acid.
Answer:
We can distinguish between an alcohol and a carboxylic acid on the basis of their reaction with carbonate and hydrogen carbonates. Acids reacts with carbonate and hydrogen carbonate to evolve CO2 gas that turns lime water milky.
Metal carbonate / metal hydrogen carbonate + carboxylic acid.
↓
Salt + water + Carbondioxide.
Alcohols on the other hand, do not react with carbonates and hydrogen carbonates.
OR
What are addition reaction? What are catalysts? Illustrate with an example.
Answer:
Reactions which involve addition of two reactants to form a single product are called addition reactions. Catalysts are the substances which can change, usually increase the speed of a chemical reaction without being used up in that reaction.
For example, vegetable oils having long unsaturated carbon chains are converted into vegetable ghee by heating them in presence of Nickel, platinum or palladium metals used as catalysts.

V. Answer the following questions. ( 4 × 4 = 16 )
Question 34.
a) Define Mendeleev’s periodic law.
Answer:
Mendeleev’s periodic law states that the properties of elements are a periodic function of their atomic masses, b) The elements of the third period of the periodic table are given below :
![]()
a) Which atom is bigger, Na or Mg? Why?
Answer:
Sodium is bigger than magnesium at it has lesser nuclear charge so there is less force of attraction between nucleus and valence electrons and less effective nuclear charge. It is, therefore bigger in size.
b) Identify the most i) Metallic and ii) Non-metallic element in period 3.
Answer:
i) Sodium is the most metallic as it can lose electrons easily due to its larger atomic size.
ii) Chlorine is the most non – metallic element because it can gain electrons easily due to its smallest atomic size.
OR
a) What are groups and period in the periodic table?
b) Two element M and N belong to group I and II respectively and are in the same period of the periodic table. How do the following properties of M and N vary?
1) Size of their atoms
2) This metallic characters
3) Their valencies informing oxides.
4) Molecular, formulae of their chlorides.
Answer:
a) The vertical columns in the periodic table are called group. The horizontal rows in the periodic table are called periods.
b) 1. M and N belong to the same period but group I and li. Therefore N will be smaller than M as a atomic size is decreases from left to right.
2. M is more metallic than N. Metalic character goes on decreasing from left to right as tendency to lose electrons.
3. Their valencies are 1 and 2 respectively in forming oxides. Valency goes on increasing first and then decreases.
4. MCl, NCl2 are molecules formulae of their chlorides.
![]()
Question 35.
Draw the diagram showing the structure of human alimentary canal and label the following parts.
a) The part which stores bile juice
b) The longest part of the alimentary
Answer:
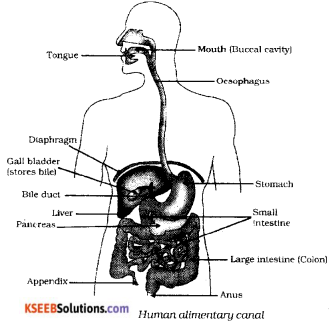
Question 36.
a) Oil and fat containing food items are flushed with nitrogen. Why?
Answer:
To prevent the oxidation of oil and fat present in food items, nitrogen gas is flushed in food packets products formed due to oxidation of oil and fat have unpleasant smell and taste due to rancidity. Flushing food items containing fat and oil with nitrogen prevents rancidity.
b) Write the balanced chemical equations for the following reactions.
Answer:
i) Calcium hydroxide + Carboridioxide → Calcium carbonate + water
Answer:
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
ii) Zinc + Silver nitrate → Zinc nitrate + silver
Answer:
Zn(s) + 2AgNO3 → Zn(NO3)2(aq) + 2Ag(s)
iii) Aluminium + Copper chloride → Aluminium chloride + Copper
Answer:
2Al(s) + 3CuCl2(aq) → 2AlCl3(aq) + 3Cu
Question 37.
a) List the physical properties of metals.
Answer:
Physical properties of metals are :
- Metals are usually hard.
- They are Sonorous.
- They are lustrus
- Metals exhibit malleability and ductility
- They exhibit high tensil strength and have high densities.
b) Differentiate between roasting and calcination. Explain the two with the help of suitable chemical equations.
Answer:
Roasting : It is a process in which sulphide ore is heated in the presence of oxygen to convert into oxide.
2Zns + 3O2 → 2ZnO + 2SO2
Calcination : It is a process in which carbonate ore is heated in the absence of air to form oxides.
![]()
By reduction process Zn can be extracted from its ore.
Reduction : 2ZnO + C → ZnO + CO2
![]()
VI. Answer the following question. ( 1 × 5 = 5 )
Question 38.
a) What is fertilisation? Distinguish between external fertilisation and internal fertilisation. What is the site of fertilisation in human beings.
Answer:
Fertilisation is defined as the fusion of a male gamete (sperm) with a female gamete (an ovum or egg) to form a zygote during sexual reproduction external fertilisation.
- The fusion of male gamete (sperm) and female gamate (ovum) occur outside the body.
- Both individuals discharge their gamates outside the body.
- Development occurs outside the body.
Ex: Frog.
Internal fertilisation :
- The fusion of gametes occurs inside the body.
- Only the male discharge sperm into female gentital tract.
- Development occurs inside the body.
Ex : Humans, cattle etc.
The site of fertilisation in human beings is in the fallopian tube of female reproductive system.
b) How does growing embryo get nutrition from the mother’s blood?
Answer:
The embryo gets nutrition from the mother’s blood with the help of a special tissue called placenta. This is a disc which is embedded in the uterine wall and transfer glucose and oxygen from the mother to the embryo.