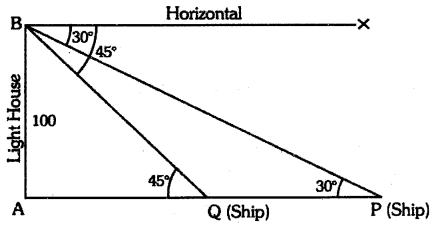
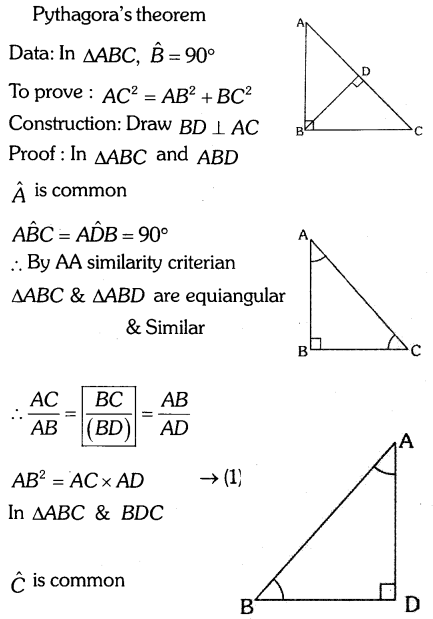
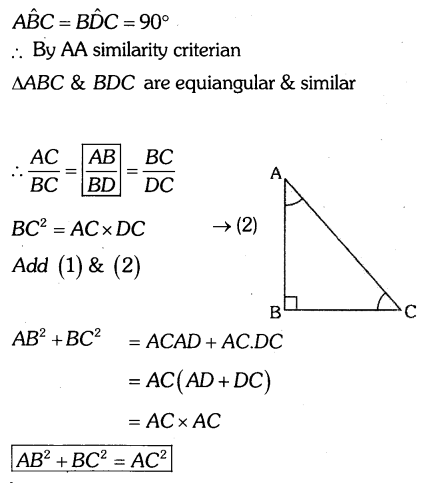
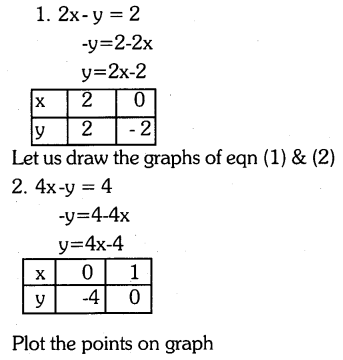
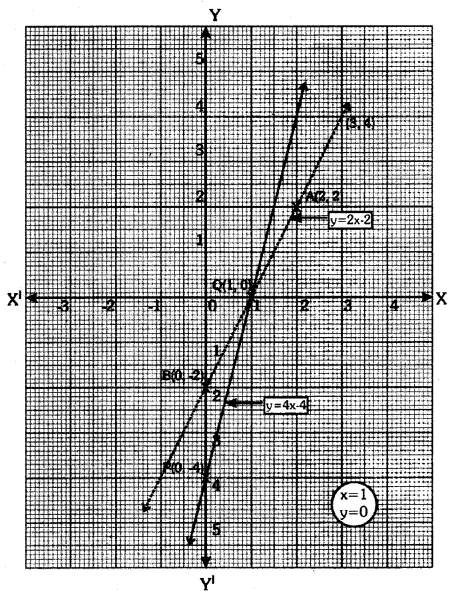
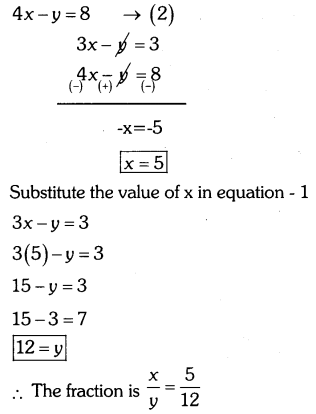
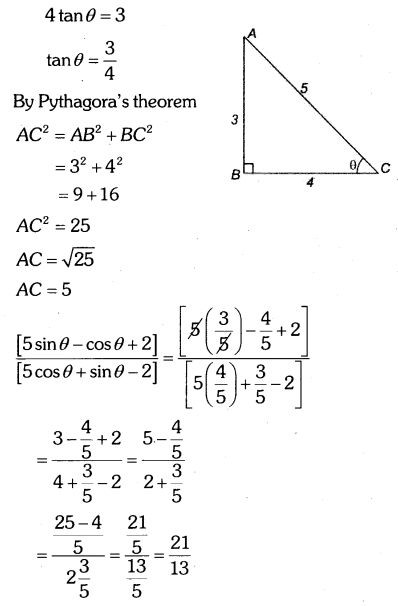
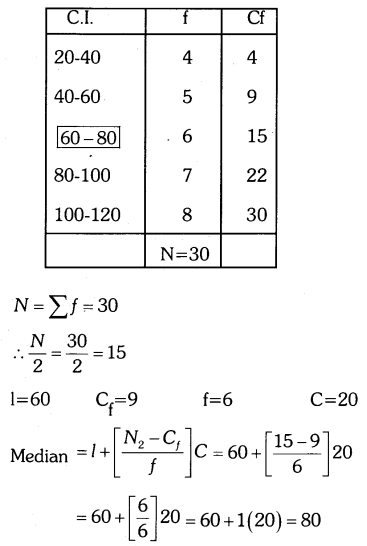
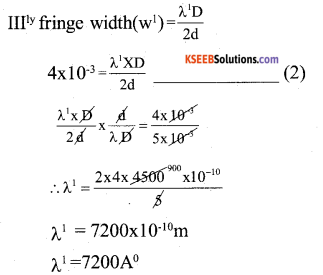
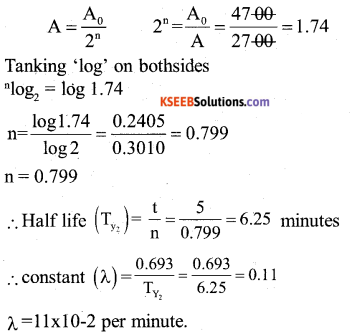
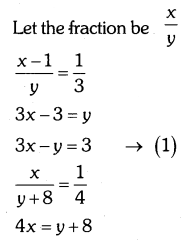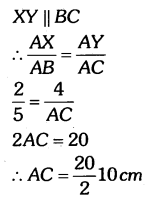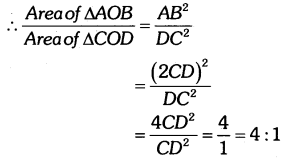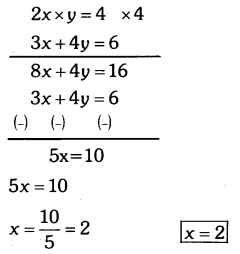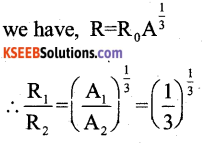By understanding the academic needs we have prepared the Karnataka State Board English Class 9 Solutions Chapter Wise. Our aim is to help the students by providing the question and answers chapter wise and help them to gain a good score in the exams. Before you start your preparation go through the chapters covered in this academic. So go through them and Download KSEEB Solutions for Class 9 English Poem Chapter 10 Photograph Question and Answers Pdf for free.
Karnataka State Board Class 9 English Poem Chapter 10 Photograph
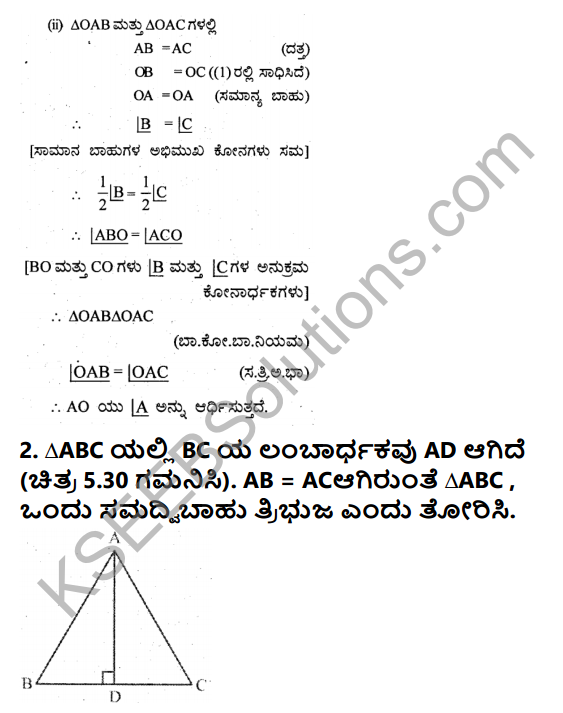
The topics covered in Karnataka Secondary Education Examination Board Class 9 Solutions for English Chapter 10 Photograph. The KSEEB Solutions Class 9 English Solutions Chapter 10 Photograph Question and Answers are prepared according to the latest edition.The Chapterwise page will help the students to revise the syllabus during the exams.
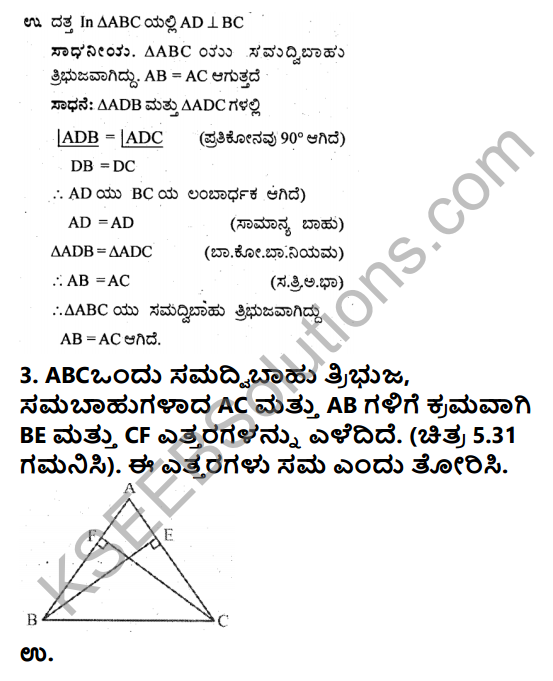
Photograph Questions and Answers, Summary, Notes
Comprehension:
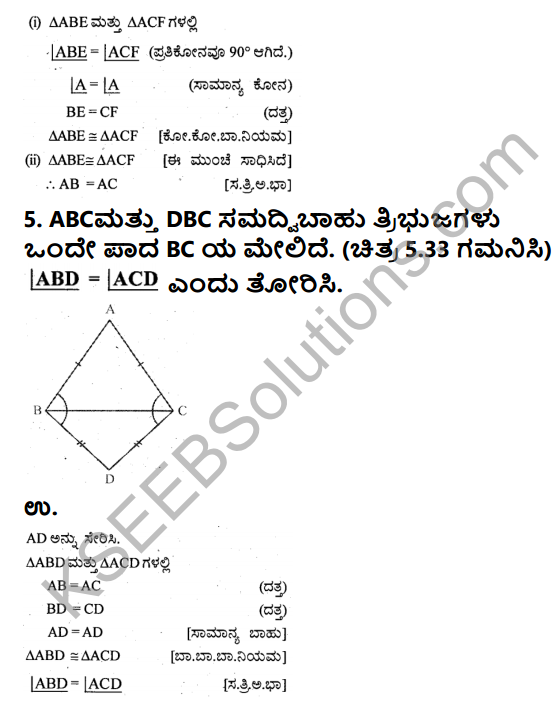
C1. Answer the following in a sentence or two each:
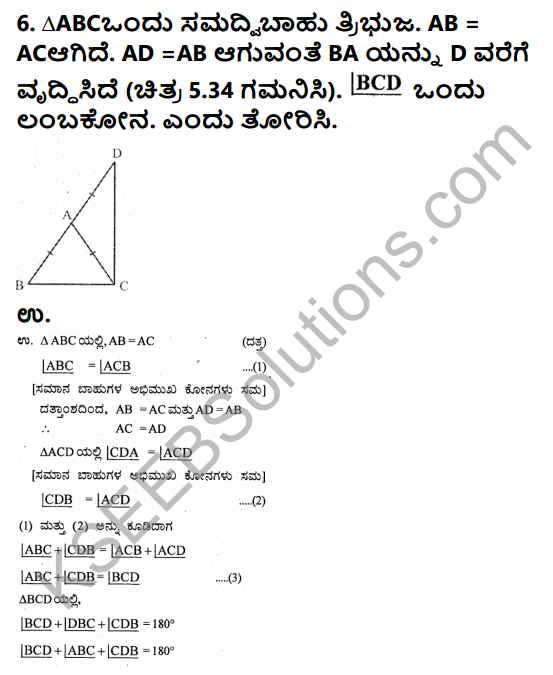
Question 1.
How many people are there in the photograph?
Answer:
There are three people in the photograph.
Question 2.
How is the poet related to the people in the photograph?
Answer:
The people in the photograph are the poet’s mother and her cousins, Betty and Dolly.
![]()
Question 3.
Who was taking the snapshot?
Answer:
An uncle was taking the snapshot.
Question 4.
Is the mother described in the photo alive?
Answer:
No, she is dead.
Question 5.
Which aspect of the mother does the poet like very much?
Answer:
The poet has sweet memories of the smiling and sweet face of the mother which she likes. This nature of the mother is captured in the photo.
C2. Answer the following:
Question 1.
Why does the writer say
“And of this circumstance
There is nothing to say at all.
Its silence silences”?
Answer:
The poet says that her mother had been dead and now she finds herself in a situation in which there is nothing to be said but only emptiness. The silence of this situation silences her. In other words, she is left speechless. Fate has killed all the feelings in her.
By the phrase ‘of this circumstance’, the poet means the circumstance of the death of her mother. The poet puns upon the. word ‘silence’. Since there are no details, what she is left with is silence and this silence silences her.
There are different possible meanings of the use of the word ‘silence’ as verb: she cannot say anything as she does not know anything about the circumstance of the death; or she does not have words to express her feelings; or the loss of her mother has stifled her voice. If we go one step further, we can even wonder whether the silence is owing to the unnatural nature of the death of the mother.
Question 2.
Does the poet notice any change in the mother after the poet was bom? What do you think could have made the change in the mother’s face?
Answer:
Yes, the poet notices the change in the mother’s face after she was born. This could have been the outcome of sorrowful incidents or hardships in life. Age and ill health also might have made the mother lose the sweetness of her face and smile.
The poet is looking at her mother’s photograph which is indeed an old one. With it she can see how her mother looked when she was a little girl of twelve. The photo shows her on a beach with her two girl cousins who are younger than her, holding her hand.
It might have been windy at that time as their hair was flying on their faces when the uncle took the photograph. All the three smile through their flying hair. Looking at the photograph, the poet says that her mother had a sweet face, but it was a time before the poet was born.
The sea was washing their feet. The poet says that the sea has changed only a little but change has come about the ones whose feet it was washing. After 30 or 40 years, the mother would take out the photograph and take a look at it.
By that time, she was married and had a daughter. She would laugh a little and say “Look at Betty and Dolly, see how they have dressed for the beach”. By now, she can only remember those days. A huge change has come about her and she is no longer that small, innocent girl of twelve. After some years, when the poet’s mother dies, for the poet, her mother’s laughter becomes a thing of the past.
That’s why she says “the sea holiday was her past and mine is her laughter”. In the same way as the mother remembers her old days, the poet can remember her mother. The poem also shows that in due course of time, the two of them learned to live with their losses though the loss had made a permanent impression on their wry faces.
![]()
Question 3.
Why are the feet described as ‘transient feet’?
Answer:
The word ‘transient’ means fleeting/ passing or temporary. It is the opposite of permanent. The ‘feet’ are described with an adjective ‘transient’ to drive home the truth that the impression they make on the sand is transitory. They get washed off by the waves.
Everything about life is transient. Single incidents, as well as whole life itself, are transient. Only nature remains as a permanent feature around us. Thus, the poet presents a contrast between human life and the sea.
The poem lends itself to multiple interpretations with regard to the transitory nature of life. If the passage of time is one aspect of the transitory nature of life, sudden unexpected occurrences can also indicate how ephemeral everything is.
We can have a different reading of the poem here. At one point the children are posing for the photograph holding their aunt’s hand. At another, the sea washes their terribly transient feet. The word ‘terribly’ makes us wonder whether the children are swept away by the waves of the sea.
C3. Answer the following questions on your own.
Question 1.
What is the mood of the poet?
Answer:
Melancholy marks the utterances of the poet. The poet has a deep sense of loss on losing her mother and the tone of sadness is all-pervasive.
Question 2.
Which line in the poem do you like the most? Why?
Answer:
I like the line ‘All three stood still to smile through their hair’. On the one hand, it creates a powerful mental picture of the three human figures against the breeze on the seashore posing for the photograph. The golden time of childhood when children are filled with mirth and are free from all worries and complexities of life is powerfully pictured here.
Smiling through the hair is typical of people who pose on the seashore. The description captures the innocuous and blithe spirit of the children which is contrasted against the care and concern of old age. It shows the poet’s power of observation and description.
The line gets connected to the title also. A photograph becomes a metaphor for all that life captures and also loses. Life is transitory and we are likely to lose many things which we will remember only through photographs.
![]()
Question 3.
Is there any change in the life of the poet’s mother over the years? What kind of a person, you think, she was? Describe the mother in the poem in your own words.
Answer:
The three stanzas of the poem depict the three stages in the life of the mother – as a child with her cousins, as a mother looking at the old photograph, and as a memory for the daughter after her death. She had a smiling and sweet face in the photograph when she posed for it, holding the hands of her cousins.
However, the poet cannot remember witnessing the same cheerfulness on the mother’s face in her recollections of her mother after she was born. What could have been the reason for the change? Apparently, the poet’s mother had posed for the photograph with her cousins when she was young and was not yet bogged down by the responsibilities and hardships of life.
As people age, along with inevitable physical changes, they also experience a change in their mental make-up because of the challenges in life. The line,’… how they dressed us for the beach,’ indicates that the mother herself was quite young and followed the directions given by others when she accompanied her cousins to the beach.
Additional Questions:
Question 1.
What does the word ‘cardboard’ denote in the poem? Why has this word been used?
Answer:
The word ‘cardboard’ means a very stiff and thick paper. Here the cardboard is a part of the frame that keeps the photograph intact. Its use in the poem is ironical. It keeps the photograph of that twelve-year-old girl safe who herself was ‘terribly transient’. She had died years ago.
Question 2.
What has not changed over the years? What does this suggest?
Answer:
The sea has not changed over the years. It brings out the ‘transient’ nature of man when compared to nature and its objects. Time spares none. The pretty faces and the feet of the three girls are ‘terribly transient’ or mortal when compared to the ageless and the unchangeable sea.
Question 3.
The poet’s mother laughed at the snapshot. What did this laugh indicate?
Answer:
The poet’s mother would laugh at the snapshot as it would revive her memories of the old happy days on the sea beach and the strange way in which they were dressed up for the beach. The laugh indicates her youthful spirit.
![]()
Question 4.
What is the meaning of the line “Both wry with the laboured ease of loss”?
Answer:
Both the poet’s mother and the poet suffer a sense of loss. The mother has lost her childlike innocence and joyful spirit that the photograph captured years ago. For the poet the smile of her mother has become a thing of the past. Ironically, both labour to bear this loss with ease.
Question 5.
What does ‘this circumstance’ refer to?
Answer:
‘This circumstance’ refers to the death of the poet’s mother. The photograph of the dead mother brings sad nostalgic feelings in the poet. But the poet has nothing to say at all about this circumstance. The silence of the poet makes the pall of silence prevailing there still deeper.
Question 6.
The three stanzas depict three different phases. What are they?
Answer:
In the first stanza, the poet’s mother is shown as a twelve-year-old girl with a pretty and smiling face. She went paddling with her two cousins. This phase is before the poet’s birth. The second phase describes the middle-aged mother laughing at her own snapshot. The third phase describes the chilling pall of silence that the death of the mother has left in the life of the poet.
Question 7.
“The sea, which appears to have changed less, washed their terribly transient feet.” – How does the poet contrast the girls’ ‘terribly transient feet’ with the sea?
Answer:
All the girls standing on the beach have ‘terribly transient’ existence. They are mortal and suffer physical changes with the passage of time. The mother’s sweet face and her smile has disappeared years later. But the vast sea remains unchanged of seems ‘to have changed less’ in their comparison.
Question 8.
‘Both wry with the laboured ease of loss’ – What is the loss? Describe the irony in the situation.
Answer:
Actually, both the poet and her mother suffer a sense of loss. The mother loses her carefree childhood. She can’t have those moments of enjoyment again that she once experienced at the beach. She can’t be a sweet smiling girl of twelve again.
This is also the poet’s loss. Perhaps she will never see that smiling face and experience her laughter again in life. The irony of the situation is that both of them struggle to bear the loss with tolerable ease.
Multiple Choice Questions:
Question 1.
The poet looks at the photograph of her mother taken when she was
A) ten years old
B) twelve years old
C) an old woman
D) a young lady
Answer:
B) twelve years old
![]()
Question 2.
The mother had gone on a sea-holiday
A) with her father
B) with her mother
C) with her cousins
D) with her daughter
Answer:
C) with her cousins
Question 3.
The photograph was taken by her
A) father
B) cousin
C) sister
D) uncle
Answer:
D) uncle
Question 4.
The Phrase ‘Their terribly transient feet’ suggests that
A) their feet were moving
B) they were padding
C) life is not permanent
D) life is everlasting
Answer:
C) life is not permanent
Question 5.
The poet’s mother is dead and the poet has nothing to say about that situation because
A) she does not want to say anything
B) the silence of death has silenced her
C) she cannot relive the past
D) she knows her mother would come back.
Answer:
B) the silence of death has silenced her
Question 6.
The mother saw the photograph after …………. years.
A) twenty or thirty
B) twelve or thirteen
C) four or five
D) one or two.
Answer:
A) twenty or thirty
![]()
Question 7.
The names of the cousins are
A) Bertha and Pinky
B) Betty and Dolly
C) Rosa and Mary
D) Laila and Pinky.
Answer:
B) Betty and Dolly
Question 8.
The cardboard shows the pictures of
A) two schoolgirls
B) two real sisters
C) two neighbours
D) narrator’s mother and her two cousins.
Answer:
D) narrator’s mother and her two cousins.
Question 9.
The ‘big girl’ here means
A) the eldest cousin of the mother
B) the tallest of the girls
C) narrator’s mother
D) the heaviest of the girls.
Answer:
C) narrator’s mother
Question 10.
The sweet face the photograph showed was that of the
A) narrator’s cousin
B) narrator’s father
C) narrator’s mother
D) narrator’s brother.
Answer:
C) narrator’s mother
Question 11.
The photograph was taken
A) when the narrator was about twelve
B) about twelve years ago
C) when the narrator was a child
D) when the narrator was not even born.
Answer:
D) when the narrator was not even born.
Question 12.
Who were ‘Betty’ and ‘Dolly’?
A) narrator’s cousins
B) her sisters
C) her mother’s cousins
D) her neighbours.
Answer:
C) her mother’s cousins
![]()
Question 13.
‘Its silence silences’ means
A) death’s silence
B) silence only brings out deeper silence
C) poet’s silence
D) silence caused by the mother’s death gives birth to a pall of silence.
Answer:
D) silence caused by the mother’s death gives birth to a pall of silence.
Photograph by Shirley Toulson About The Poet:
Shirley Toulson was born in 1924 in Henley-on-Thames, England. She had a huge passion for writing and was greatly influenced by her father who was a writer too. She secured a B.A. in Literature from Brockenhurst College in London in the year 1953. Shortly, she took writing as a career but meantime also served as editor for many magazines.
Celtic Christianity, influenced her so greatly that most of her major works such as ‘Celtic Alternative’ in 1987 and ‘Celtic Year’ in 1993 were on that topic. But a whole lot of other works, including collections of poetry, essays on education and other publications on diverse topics brought her even more fame.
Photograph Summary in English
The poem ‘Photograph’ is an interesting piece describing how a photograph can set in motion recollections of different kinds. The photograph is also an indicator to the fact that everything in life is transitory. What is captured years ago on the photograph is a contrast to what people turn out to be years later.
The photograph can also be illusory in nature as it might not show exactly how the people being featured in the photograph felt at that particular point of time. The photograph can also refer to our desperate attempts to defy the passage of time and the inevitable changes that take place along with the passage of time.
The speaker begins the poem by saying, ‘A photograph: The cardboard shows me how it was.’ The photograph is of the speaker’s mother with her cousins. The photograph features the two girls holding the hands of the speaker’s mother and the speaker knows that the photograph was taken by an uncle. The speaker is struck by the realisation that her mother was sweet in her looks at the time the photo was taken.
This was even before the speaker was 4}orn. While referring to the sea washing the terribly transient feet of the people who posed for the photograph, the speaker introduces a contrast between the sea and the people.
People change with the passage of time, but the sea seems to have changed very little. This is an indicator to the permanence of nature and transience of man. Unfortunately, the change in human beings, both physically and emotionally, is for the worse.
The smiling, sweet face of the young girl is replaced by the melancholic face of the mother. Just as physical changes are inescapable, even mental deterioration also seems to be inevitable.
Twenty or thirty years passed since that photograph had been taken. Whenever mother looked at the photograph, she would laugh. She would point out to Dolly and Betty how they were dressed up (by their parents) for the sea-holiday.
That sea-holiday had become a thing of the past for the mother. And that sweet laughter of the mother which the photograph had captured had become the poet’s own past. Both of them suffered from a sense of loss. Ironically, both of them were labouring to ease the loss.
The poet says that her mother has been dead for years. She has been dead nearly as many years as she had lived. She (the poet) has nothing to say about the circumstances of her death or about the situation captured in the photo. Silence only brings out deeper silence and makes things mysterious.
The photograph describes three stages. In the first stage, the photograph shows the poet’s mother standing on the beach enjoying her holiday with her two girl cousins. She was 12 or so at that time.
The second stage takes us twenty or thirty years later. The mother would laugh at the way she and her cousins Betty and Dolly were dressed up for the sea-holidays. In the third stage, the poet remembers the mother with a heavy heart as the mother is already dead. The photograph revives a nostalgic feeling in the poet.
Thus, through an everyday, commonplace incident of a photo being clicked on the seashore, the poet is able to drive home philosophical reflections on the very nature of life.
Glossary:
paddling: moving like rowing.
transient: momentary; not lasting for long
wry: distorted.
We hope the information prevailed in this article is helpful for all the students of Class 9. The Karnataka State Board Solutions for Class 9 English Chapter 10 Photograph Question and Answers pdf enhance your skills and score good marks in the exams. Stay tuned to get the latest information about the KSEEB Solutions Class 9 English Solutions.