Students can Download 1st PUC Chemistry Model Question Paper 5 with Answers, Karnataka 1st PUC Chemistry Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka 1st PUC Chemistry Model Question Paper 5 with Answers (Old Pattern)
Time: 3.15 Hours
Max Marks: 70
Instructions:
- The questions paper has four parts A, B, C and D.
- In part A each question carries ONE Marks. In part B, each question carries TWO marks, in part C each question carries FIVE marks. In Part D-1 carries TEN Marks and each question in D -2 carries FIVE marks.
- Write balanced chemical equations and draw diagram wherever necessary.
Part – A
I. Answer of all the following questions: ( 1 × 9 = 9 )
Question 1.
What is a limiting reagent in a reaction ?
Answer:
The reactant which gets completely consumed or limits the amount of product formed in a reaction
Question 2.
State the law of conservation of mass.
Answer:
It states that matter can neither be created nor be destroyed
Question 3.
Select an isoelectronic pair among the following Na+,Cl–,F–,Li+
Answer:
Na+ ,F–
![]()
Question 4.
What is the effect of temperature on Viscosity of liquids ?
Answer:
Viscosity of liquids decrease as temperature rises.
Question 5.
A closed thermos flask containing hot coffee represents what type of a system ?
Answer:
Isolated.
Question 6.
Write the expression for Kc for the equilibirium, N2(g) + 3H2(g) ⇌ 2NH2(g)
Answer:
\(\mathrm{K}_{\mathrm{c}}=\frac{\left[\mathrm{NH}_{3}\right]^{2}}{\left[\mathrm{N}_{2}\right]^{1}}\)
Question 7.
H2+and H2–have same bond order but H2+is more stable than H2–, why ?
Answer:
H2–has en electron in antibonding MO which makes it less stable than H2+
Question 8.
What volume of O2 is produced by 1 litre of “10 volume” H2O2 at STP ?
Answer:
10 litres.
Question 9.
Name the alkali metal which is radioactive ?
Answer:
Francium (Fr)
Question 10.
Which element is diagonally related to lithium ?
Answer:
Magnesium (Mg)
![]()
Question 11.
Give an example for a Heterocyclic compound.
Answer:
Pyridine, Furan, Thiophene.
Part – B
II. Answer any Five questions: ( 5 × 2 = 10 )
Question 12.
Write balanced chemical equation for the combustion of methane. How many mole of methane are required to produced 88g Carbon dioxide by the combustion of Methene ?
Answer:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
\(\begin{array}{cc}{12+4 \times 1} & {12+2 \times 16} \\{16 g} & {44 g}\end{array}\)
44g of CO2 requires 16g of CH4.
88g of CO2 requires ?
= \(\frac{88 \times 16}{44}\) = 32g of CH4 = 2 mole of methane.
Question 13.
Size of Na+ ion is smaller than Na. Give reason.
Answer:
During the formation of Na+ ions effective nuclear charge increases thus electron cloud shrinks.
Question 14.
What causes temporary and permanent hardness of water ?
Answer:
Temporary Hardness – presence of dissolved Bicarbonates of Mg and Ca.
Permanent Hardness – Presence of dissolved Chlorides and sulphates of Mg and Ca.
![]()
Question 15.
Derive Ideal gas equation using gas laws.
Answer:
From Boyle’s law V ∝ \(\frac{1}{p}\) ………..(1)
From Charle’s law V ∝ T ………..(2)
From(1) and (2)
V ∝ \(\frac{T}{p}\)
PV ∝ T
Or PV – RT
Question 16.
(i). Chemical equilibrium is dynamic. Give reason.
Answer:
Both forward and backward reactions are occurring at the same rate.
(ii). What is the effect of a Catalyst on the equilibrium of a reversible reaction. 2
Answer:
A catalyst has no effect on the position of equilibrium but it helps the reaction to attain equilibrium quickly.
Question 17.
Diamond is a bad conductor of electricity but Graphite is a good conductor. Justify the statement.
Answer:
Due to sp3 hybridization in Diamond no free electrons are present.
In Graphite due to sp3 hybridization, there are free electrons to conduct electricity.
![]()
Question 18.
Write any two common chemicals of photochemical smog.
Answer:
Ozone, Nitric oxide, Acrolein, Frmaldehyde and peroxy acetyl nitrate.
Part – C
Note : Answer any Four questions: ( 4 × 4 = 16 )
Question 19.
a. Define mole fraction.
Answer:
It is ratio of number of moles of a particular component to the total number of moles
of the solution. Mole fraction = \(\frac{\mathbf{n}_{\mathrm{B}}}{\mathbf{n}_{\mathrm{A}}+\mathbf{n}_{\mathrm{B}}}\)
b. Calculate the mass percent of the solute, if 4 g of a solute is dissolved in 18g of water.
Answer:

Question 20.
a. Explain the formation of line spectrum of Hydrogen based on Bohr’s atomic theory. Write the energy level diagram for Lyman and Balmer series of the hydrogen spectrum.
Answer:
According to Bohr’s Atomic model:
A sample of H2 gas contains large number of hydrogen molecules. When electric discharge is passed through hydrogen gas the molecules absorbs energy and split up into atoms. Now the electron present in different atoms absorbs different energy and jump to different high energy state. The electrons in the higher energy is unstable thus returns back by emitting radiations having different wavelength. Hydrogen spectrum consists of many spectral lines event through the hydrogen only one electron.
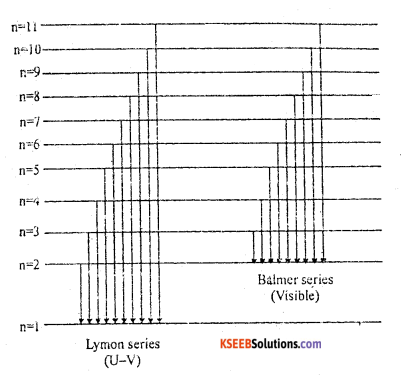
Lymon series : The lines obtained when and electron jumps from 2nd, 3rd, 4th…. i.e., from any higher energy level to first energy level (n1 = 1) are called Lyman series. These lines are found in ultraviolet region.
Balmer series : The lines obtained when electrons jumps from 3rd, 4th 5th… from any higher energy level to second energy level are called Balmer series. This is found in visible region.
Or (Internal choice)
b. (i) Calculate the wavelength of a ball of mass 0.05 kg moving with a velocity of 5ms-1. Given : h = 6.626 × 10-34 Js. 2+2
Answer:
![]()
![]()
Question 21.
a. Classify the following physical quantities having α Extensive and Intensive properties Mass, Density, Temperature and Heat capacity.
Answer:
Extensive property – Mass, Heat capacity
Intensive property – Density, Temperature
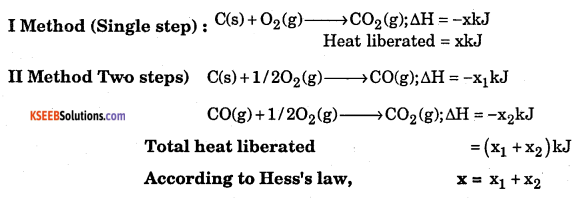
b. State Hess’s law of constant summation.
Answer:
Hess’s law : .Whether a chemical reaction takes place in a single step or in several steps, the total change in enthalpy remains the same. Consider the formation of CO2 from C.
Question 22.
Using Le-Chatelier’s principle explain the effect of
(i) Addition of CH4 (ii) Addition of CS2 (iii) Removal of S2 (iv) Removal of H2S on the equilibrium,
CH4(g) + 2S2(g) ⇌ CS2(g) + 2H2S(g) 4
Answer:
i) Increase in concentration of CH4 , favours forward reaction.
ii) Increase in concentration of S2, favours backward reaction.
iii) Decrease in concentration S2, favours backward reaction.
iv) Decrease in concentration of H2S , favours forward reaction.
Question 23.
a. Give the composition of Washing soda. An aquous solution of washing soda is alkaline, Give reason.
b. What happens when Cl2 (Chlorine) gas is passed into milk of Lime ? Write the equation. 2+2
Answer:
Bleaching powder is formed.
2Ca(OH)2 + 2Cl2 → CaCl2 + Ca(OCl)2 + 2H2O
![]()
Question 24.
Draw a neat labeled diagram and give the calculations involved in the estimation of Nitrogen in an Organic compound by Duma’s method.
Answer:
Principle : The organic compound containing nitrogen when heated with excess of copper oxide in the atmosphere of carbon dioxide, yields nitrogen in addition to carbon dioxide and water.
Traces of nitrogen oxides formed during combustion of organic compound are reduced to nitrogen by passing the gaseous mixture over a heated copper gauze. The percentage of nitrogen present in a given organic compound is calculated from the volume of nitrogen collected over potassium hydroxide solution from a known mass of organic compound.
Procedure : The apparatus used for the estimation of nitrogen by this method is shown in the figure.
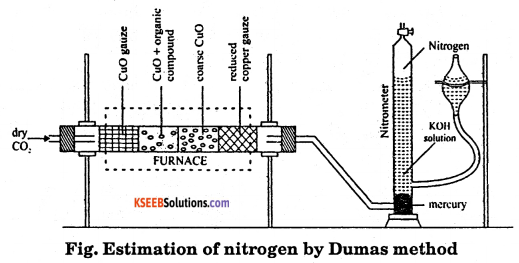
A known mass of organic compound is mixed with copper oxide and placed in the combustion tube. The carbon dioxide gas is passed through the combustion tube to displace air present in the tube. The combustion tube is now heated in the furnace. the nitrogen evolved collects in the nitrometer. The volume of the nitrogen collected is recorded after adjusting the levels of potassium hydroxide solution in the two limbs are equal. Room temperature and atmosphere pressure are recorded.
Calculation:
Mass of organic compound = mg
Volume of nitrogen in nitrometer = V cm3
Room temperature = t° C = (273 + t) K
Atmosphere pressure = P1 mm
Aqueous tension at room temperature = P′ mm
Pressure of dry nitrogen gas formed = P = (P – P′) mm
Volume of nitrogen at STP (V0) = \(\frac{\mathrm{PV} \times 273}{760 \times(273+\mathrm{t})} \mathrm{cm}^{3}\)
22,400 cm3 of nitrogen of STP = 28 g of nitrogen
Mass of V0 cm3 of nitrogen = \(\frac{28 \times V}{22,400} g\)
Percentage of nitrogen =\(\frac{28 \times V_{0} \times 100}{22,400 \times m}\)
Part – D
Note : Answer any Four questions : ( 2 × 5 = 10 )
Question 25.
a. (i) Name the isotope of Hydrogen containing two neutrons.
(ii) Mention any two limitations of Bohr’s atomic theory.
b. Atomic number and Mass number of an element are 29 & 64. How many protons and Neutrons are present in it? Why the Electronic configuration of the element.
Answer:
a) i) Tritium (1T3)
ii) It fails to account for the finer details of the hydrogen atom spectrum observed by using sophisticated spectroscopic technique, i.e., stark effect and zeeman effect.
b) It could not explain the ability of atoms to form molecules by chemical bonds.
![]()
Question 26.
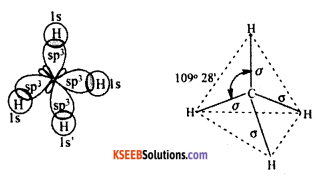
a. Explain the shape of Ammonia molecule using VSEPR theory.
Answer:
Ammonia (NH3)
Electronic configuration of N: 1s2 2s2 2p3
Valance orbital representation
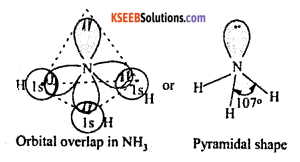
- when 2s2 orbital hybridised with 2px, 2py, and 2pz forms the following sp3 hybridised structure.
- In 2s2 orbital, the electrons are paired up hence they are not involved in bond formation called loan paired of electron (L.P).
- When sp3 hybridized N combines with 3 hydrogen atom, forms pyramidal structure of ammonia.
Pyramidal structure angle = 107°
Or (Internal Choice)
Mention any three postulates of Molecular Orbital Theory.
Answer:
- The electrons in an atom are found in atomic orbitals, the electrons in a molecule are found in molecular orbitals.
- The molecular orbitals are formed by the combination of atomic orbitals of comparable energies and proper symmetry.
- The BMO has lower energy and hence greater stability than the corresponding ABMO.
- The molecular orbitals are filled by electrons in accordance with Aufbau principle, Pauli’s exclusion principle and the Hund’s rule.
b. Lithium molecule has 4 electrons in bonding molecular orbital and 2 electrons in anti bonding molecular orbital. Write the electronic configuration and calculate the bond order of Lithium molecule.
Answer:
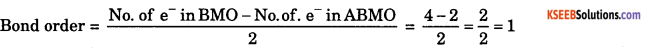
Or (Internal choice)
Calculate the Bond order, show that Helium Molecule (He2) does not exist.
Answer:
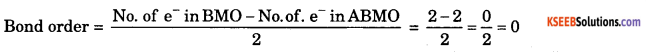
Reason: since bond order is 0 the molecule does not exist.
Question 27.
a. For the oxidation of Iron : 4Fe(s) + 3O2(g) → 2Fe2O3(g)
Entropy change and Enthalpy change at 298 K are – 549.4 J/K/mol and -1648 × 103 J/mol respectively. Calculate the free energy change for the reaction. Is the reaction spontaneous at 298 K.
Answer:
∆G = ∆H – T∆S
∆G = -1648000 + 163721.2
= -1484278.8 J/mol
=-1484.2788 kjmol
The reaction is spontaneous.
b. Define Enthalpy of solution.
Answer:
The enthalpy of solution at infinite dilution is the enthalpy change observed an dissolving the substance in an infinite amount of solvent when the interations between the ions are negligible.
![]()
Question 28.
a. In the Galvanic cell, the cell reaction is Zn(s) + 2Ag(aq)+ → Zn2++ 2Ag(a)
(i) Which of the electrode is the Anode ?
(ii) Write the Cathodic and Anodic reactions.
(iii) Identify the oxidizing and reducing agents.
Answer:
i) Zn elelctrode.
ii) Cathodic reaction : Ag++e– → Ag
Anodic rection : Zn – 2e– → Zn2+
iii) Oxidising agent: Ag+
Reducing agent: Zn
b. Show that the reaction CaCO3 → CaO + CO2 is not a Redox reaction. 3+2
Answer:
![]()
Since there is not change in oxidation state of any element before and after the reaction. It is not a redox reaction.
Question 29.
a. Explain the Ampoteric nature of Aluminium, give equations.
Answer:
Aluminium reacts both with acid and base so it behaves as amphoteric nature.
2Al(s) + 6HCl(aq) → 2Al3+(aq) + 6Cl–(aq) + 3H2(g) behaving as acid.
2Al(s) + 2NaOH(aq) + 6H2O(l) → 2Na+[Al(OH)4]– (aq) + 3H2(g) behaving as base.
b. Name the type of hybridization of
(i) Boron in Diborane
(ii) Carbon in Graphite
Answer:
i) sp3 hybridization
ii) sp2 hybridisation
Question 30.
a. Explain the mechanism of chlorination of Methane, with equations.
Answer:
Mechanism of chlorination of methane involves three types.
Step 1 : Initiation : Chlorine absorbs energy and undergoes homolysis to give chlorine free radicals.
![]()
Step 2 : Propagation : Chlorine free radical reacts with methane to give methyl free radical.
Cl• + CH4 → CH3•+ HCl
The methyl free radical reacts with chlorine to form methyl chloride and chlorine free radical.
CH3•+ Cl2 → CH3Cl + Cl•
Step 3 : Termination : Free radicals combine to form stable products.
Cl• + Cl• → Cl2 (Chlorine)
CH3•+ Cl• → C2H6 (Ethane)
CH3• + Cl• → CH3Cl (Methyl Chloride)
b. How do you convert Benzene to Benzene hexachloride (BHC) ? 3+2
Answer:
![]()
Under ultra violet light, three chlorine molecules add to benzene to produce benzene hexa chloride, C6H6Cl2 which is also called gammaxane.
Part – E
Answer any Three of the following questions: ( 3 5 = 15 )
Question 31.
a. (i) Explain why BF3 molecule has zero dipole moment although the B-F bonds are polar ?
(ii) Alkali metals and Halogens form Ionic compounds. Give reason.
Answer:
i) Shape of BF3 is Trigonal planar.
The net sum of bond moment is equal to zero because of cancellation of bond moments due to symmetry in the molecule.
ii) Alkali metals have low ionization enthalpy hence they easily form cations.
Halogens have high electron gain enthalpy hence they easily form Anions.
b. What is Hydrogen bonding ? Sketch the Hydrogen bonding in liquid Hydrogen fluoride ? 3+2
Answer:
It is an electrostatic force of attraction between an electropositive element and electronegative element Hs+-Fs.
![]()
Question 32.
a. Write the expression for the compressibility factor (Z) for one mole of a gas and name the terms in it. What is the value of Z for an ideal gas ?
Answer:
z = \(\frac{p v}{R T}\) z = compressibility, P = pressure, V = Volume, T = Temperature in K, Z = 1 for ideal gas, R = Universal gas constant.
b.A balloon is filled with Hydrogen gas at room temperature. It will burst if pressure exceeds 0.2 bar. If at 1 bar pressure the gas occupies 2.27 liter volume, up to what volume can the balloon be inflated ?
Answer:
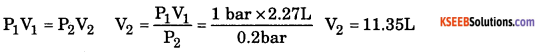
Question 33.
a. What is a Bronsted – Lowery Acids ? Identify the two Conjugate acid-base pairs in the following.
NH3 + HCl ⇌ NH4++ Cl–
Answer:
Bronsted – lowery theory
An acid is substance which donates proton base is a substance which accepts a portion so this theory is also called as proton theory.
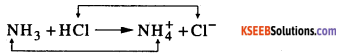
Conjugate acid base pair
b. Calculate the solubility of Lead Chloride at 298. K, if its solubility product is 1.6 × 10-5.
Ans: For PbCl2ksp = 4s3
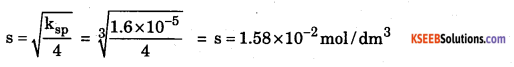
Question 34.
a. Write any two differences between Resonance effect and Electromeric effect.
Answer:
| Resonance Effect | Electromeric Effect |
| 1. It takes place in conjugated double bond system | 1. It takes place in the molecule having c = c |
| 2. It is a permanent effect takes place in absence of attacking reagent. | 2. It is temporary effect takes place in presence of attacking reagent. |
b. What type of structural isomerism is exhibited by the Propanone and Propanal pair ?
Write their structural and identify the functional groups present in them. 2+3
Answer:
Functional isomerism
Structure : CH3 – CO – CH3, CH3 – CH2 – CHO
Functional groups : Ketone (C = O); Aldehyde (-CHO)
![]()
Question 35.
a. (i) Name the chief product obtained when Benzene is heated with concentrated Sulphuric acid. Give equation ?
Answer:
Benzene Sulphonic Acid
Suiphonation : Benzene reacts with concentrated sulphuric acid at 80°C to form benzene suiphoric acid.
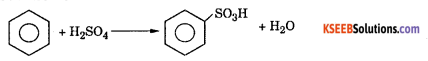
Mechanism : This involves the following steps.
Step 1 : Generation of electrophile SO3.
2H2SO4 ⇌ SO3 + H3O+ + HSO4–
Step 2 : The electrophile SO3 attacks tihe benzene ring to form a carbocation
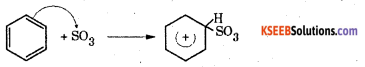
Step 3 : Loss of a proton to form a sulphonate ion. The proton is removed by HSO4–
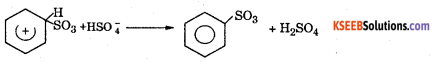
Step 4 : Addition of proton to give benzene sulphonic acid.
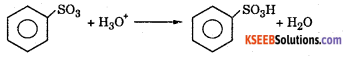
(ii)Write the resonance hybrid structured of Benzene.
Answer:

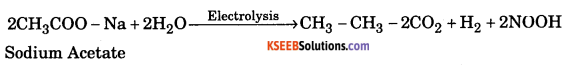
b. How is Ethane prepared from sodium acetate by electrolytic method ? 2+1+2
Answer:
Karnataka 1st PUC Chemistry Model Question Paper 1 with Answers
Time: 3.15 Hours
Max Marks: 70
Instruction:
- The questions paper has five parts A, B, C, D and E. All parts are compulsory.
- Write balanced chemical equations and draw labeled diagram wherever allowed.
- Use log tables and simple calculations f necessary (use of scientific calculations is not allowed).
Part – A
Note : Answer any nine questions: ( 9 × 1 = 9 )
Question 1.
Define Molarity of a solution.
Answer:
Number of moles of solute in one litre of the solution.
Question 2.
Name the element having highest value for its Electron gain enthiaply.
Answer:
Chlorine
Question 3.
On what parameter do the Elements are classified in the modern periodic table?
Answer:
Atomic number
![]()
Question 4.
What type of Van der waals force exists between HCI molecules?
Answer:
Dipole – dipole interaction
Question 5.
What is the relationship between ∆H and ∆U for the reaction PCl5(g) ⇌ PCl3(g) + Cl2(g) ?
Answer:
∆H = ∆U + RT
Question 6.
The value of Ionic Product of water at 298 K is 1 × 10-14, what is its [H+] ?
Answer:
1 × 10-7 or 10-7 Mole/dm3
Question 7.
Name any two gases responsible for Green house effect.
Answer:
Any two gases (CH4, CO2, NO2 etc.)
![]()
Question 8.
What is the role of Heavy water in a nuclear reactor?
Answer:
As a moderator Or To slow down fast moving neutrons.
Question 9.
What is Catenation?
Answer:
The tendency of Carbon atoms to link with one another through covalent bonds to form chains and rings.
Question 10.
What type of structural isomerism is shown by the pair : But-1-yne and But-2-yn?
Answer:
Position isomerism.
![]()
Question 11.
Which one of the following acids is not present in Acid rain?
Answer:
H – COOH
Part – B
Note : Answer any Five questions: ( 5 × 2 = 10 )
Question 12.
Express i) 5 litre of milk in cubic meter (m3) ii) 25° C in Kelvin. ( 1 + 1 )
Answer:
i) 5 10-3 m-3 …………1 ii) 298 K ………..1
Question 13.
Which of the following species are Isoelectronic with Ne ? ( 2 )
(Atomic no. of Ne = 10). N3-,Na+,Al3+,Ar,Rb+ & F–
Answer:
All elements contains total 10 electrons.
![]()
Question 14.
Mention the type of Hydrogen bond in the following compounds. ( 1 + 1 )
i) Water ii) ortho-Nitrophenol
Answer:
i) Inter molecular hydrogen bonding …………1
ii) Intra molecular hydrogen bonding ………..1
Question 15.
Write Vander Walls equation for one mole of a gas and name any two terms in it. ( 1 + 1 )
Answer:

Question 16.
Calculate the pH of a solution whose Hydrogen ion conc. is 1.5 × 103 M. ( 2 )
Answer:
pH = – log[H+]
pH = – log(1.5 × 10-3)
pH = 3 – log 1.5 = 3 – 0.1761
pH = 2.8239
Question 17.
What is the composition of Permutit (Zeolite) ? How does it works in softening of hard water? ( 1 + 1 )
Answer:
Hydrated sodium aluminium silicate / Sodium aluminium silicate ………..(1) It exchanges Mg2+ and Ca2+ ions for its Na+ hence soften the hard water …. (1)
![]()
Question 18.
Explain the action of heat on Orthoboric acid. Give example. ( 1 + 1 )
Answer:
When hydro basic acid is heated two moles of water is liberated as follows.
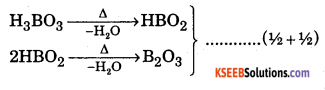
Part – C
Note : Answer any Four questions: ( 4 × 4 = 16 )
Question 19.
Mention one significance for each of the four quantum numbers. ( 4 )
Answer:
n = size of the atom
l = orientation, shape
m = subduell (s, p, d, f)
s = Spin of electrons
Question 20.
Write the Electronic configuration, Energy level diagram for the molecular orbitals of Oxygen molecule (O2). Calculate its bond order and give reason as to why O2 is paramagnetic. ( 4 )
Answer:
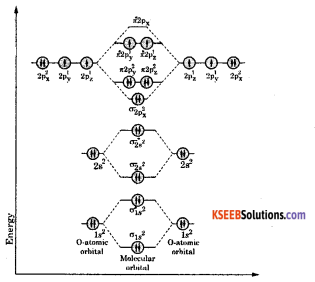
Atomic number of oxygen = 8. Electronic configuration of oxygen = 1s22s22p4. When two oxygen atoms combines, the molecular orbital energy level diagram is as shown in the figure.
From the diagram, the molecular electronic configuration of oxygen is
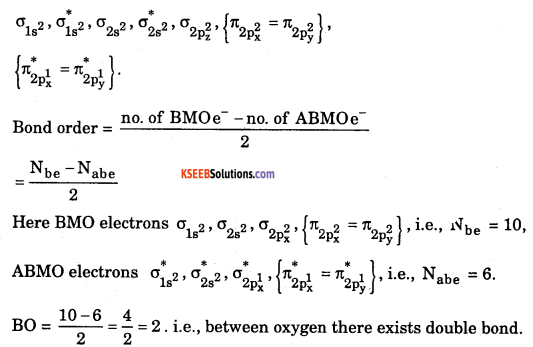
Magnetic property: There are two unpaired electrons {\(\pi_{2 \mathrm{p}_{\mathrm{x}}^{1}}^{*}=\pi_{2 \mathrm{p}_{\mathrm{y}}^{1}}^{*}\)} therefore, oxygen molecule is a paramagnetic in nature.
![]()
Question 21.
Calculate the heat of reaction of the following reaction :
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(g) ; ΔH = ?
C(graphite) + O2(g) → CO2(g); ΔH = -395.0 kJ … (1)
H2(g) + \(\frac{1}{2}\)O(g) → H2O(l); ΔH = -269.5 kJ … (2)
6C(graphite) + 6H2(g) + O2(g) → C6H12O6; ΔH = -1169.8 kJ … (3)
Answer:
Multiplying equation (1) and (2) each by 6 reversing (3), we get,
6C(graphite) + 6O2(g) → 6CO2(g); ΔH = -2370 kJ …(4)
6H2(g) + 3O2(g) → 6H2O(l); ΔH = -1616.4 kJ … (5)
C6H12O6(s) → 6C(graphite) + 3O2(g) ; ΔH = +1169.8 kJ …..(6)
Adding (4), (5) and (6),
C6H12O6(s) +6O2(g) → 6CO2(g) +6H2O(g) ;
ΔHg (C6H12O6) = -2370.0 – 1616.4 + 1169.8 = -2816.6 kJ
Question 22.
A buffer solution of pH 8.3 is prepared from ammonium chloride and ammonium hydroxide. Dissociation constant of ammonium hydroxide is 1.8 × 10-5. What is the mole proportion of ammonium chloride and ammonium hydroxide?
Answer:
pOH= 14 – pH =14 – 8.3 = 5.7
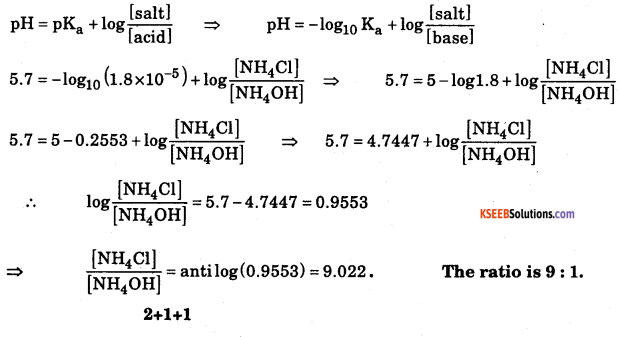
![]()
Question 23
a. A coulourless liquid ‘A’ contains H and O elements only. It decomposes slowly on exposure to light. It is stabilized by mixing urea to store in the presence of light, (i) Suggest possible structure of A. (ii) Write chemical equations for its decomposition reaction in light. ( 2 + 2 )
Answer:
The liquid A is hydrogen peroxide (H2O2)
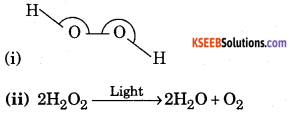
b. Give three uses of different allotropic forms of carbon.
Answer:
| Forms of carbon | Uses |
| Diamond | Gemstone, cutting, drilling, grinding, polishing, industry. |
| Graphite | Reducing agent, refractories, pencils, high temperature, crucibles, electrode making, moderator in nuclear reactors, high strength composite materials. |
| Activated carbon | Rubber industry, pigments in ink, paints and plastics |
| Coke | Fuel, strut manufacture |
| Charcoal | Fuel, reducing agent, Adsorption. |
![]()
Question 24
a. Explain the mechanism of Halogenation or chlorination of benzene. ( 3 + 1 )
Answer:
Halogenation: Benzene reacts with chlorine in the presence of FeCl3 or AlCl3 to form chlorobenzene.
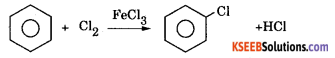
Machanism : This involves the following steps.
Step 1: Generation of electrohile Cl + Cl – Cl + FeCl3 → Cl++ FeCl4–
Step 2 : The electrophile Cl+ attacks benzene ring to form a carbon cation which is resonance stabilised.
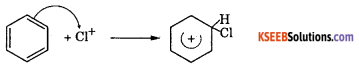
Step 3: Loss of a proton to give chiorobenzene. The proton is removed by FeCl4.
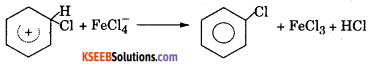
b. Name the product obtained when Benzene is hydrogenated in presence of heated Nickel catalyst.
Answer:
Cyclohexane (1)
Part – D
Note : Answer any Four questions: ( 4 × 5 = 20 )
Question 25
a. i) What is the mass percentage of Carbon in Methane ( CH4 ) (Molecular mass of CH4 =16)
Answer:
\(\frac{12}{16}\) × 100 = 75% or 75% …….. (1)
ii) Expess 9.8 g H2SO4 in mole (Molecular mass of H2SO4 = 98)
Answer:
9.8 g H2SO4 = 0.1 mole or 0.1 mole …. (1)
iii) What mass of Calcium carbonate is to be decomposed to obtain 4.4 of CO2 in the following reaction
CaCO3 → CaO + CO2 (Molecular mass of CaCO3 = 100)
Answer:
100 × \(\frac{4.4}{44}\) = 10g or g …… (1)
b. State Avegadro’s law.
How many atoms of Hydrogen are present in 1 mole of water?
Answer:
Under the same temperature & pressure equal volume of gases contains equal number of molecules.
= 2 × 6.022 × 1023 atoms …….. (1)
![]()
Question 26
a. The average kinetic energy of a gas molecule at O °C is 5.621 × 10-27 J. Calculate Boltzmann constant. Also calculate the number of molecules present in one mole of the gas.
Answer:
Average kinetic energy KE = \(\frac{3}{2}\)KT

No. of molecules in 1 mole of the gas (Avagadro’s no.) = \(\frac{R}{k}\)
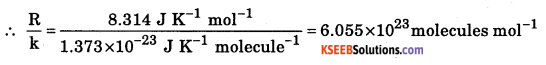
b. How vapour pressure of liquid is related to (i) temperature, (ii) nature of liquid, (iii) boiling point, (iv) atmospheric pressure?
Answer:
(i) Vapour pressure is directly proportional to temperature.
(ii) If intermolecular force of attraction is less in the liquid, its vapour pressure will be high.
(iii) Higher the vapour pressure, lower will be boiling point.
(iv) If atmospheric pressure is low, boiling point will be less and higher will be the vapour pressure.
Question 27
a. What happens to the solubility of BaSO4 when a few drops of BaCl2 solution is added to its saturated solution ? Give reason.
Answer:
Solubility decreases, Due to common ion effect
b. What is meant by Conjugate acid-base pair ?
Write the conjugate acids for CN– & H2O.
Answer:
Acid base pair which differ by one photon.
Conjugate acid of CN– is HCN ; Conjugate acid of H2O is H3O+
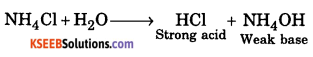
c. An aqueous solution of NH4Cl is acidic. Give reason.
Answer:
Due to hydrolysis produce HCl, reaction
![]()
Question 28
a. Give reason for diagonal relationship of lithium with magnesium.
Answer:
Both Lithium and magnesium have small size and high charge density. The electronegativities of Li is 1.0 and Mg is 1.2. They are low and almost same. Their ionic radii are similar. Hence they show similarities which is known as diagonal relationship between first element of a group with the second element in the next higher group.
b. What is the effect of heat on the following compounds ?
(a) Magnesium chloride hexahydrate
(b) Gypsum
(c) Magnesium sulphate heptahydrate
Answer:
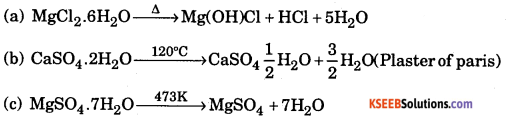
c. Between LiCl and NaCl, LiCl is soluble in Ethanol but NaCl does not give reason.
Answer:
Because LiCl is covalent compound hence dissolves in organic solvent (Ethanol) but NaCl is ionic compound hence insoluble in ethanol.
Question 29
a. How is the estimation of halogens by Carius method ? ( 4 )
b. Which method is employed for the estimation of carbon and hydrogen organic compounds ?
Answer:
Liebig’s method.
![]()
Question 30
a. What is Wurtz reaction ? Give example. ( 2 )
Answer:
When alkyl halides are heated with sodium metal in ether medium higher alkanes are formed. This reaction is known as Wurtz reaction and employed for the synthesis of higher alkanes containing even number of carbon atoms.
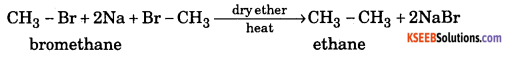
b. How is Methane prepared from sodium acetate ? ( 2 )
Answer:
Decarboxylation : When sodium salt of carboxylic acid is heated with soda lime (mixture of sodium hydroxide and calcium oxide), an alkane containing one carbon atom less them parent carboxylic acid is formed. This reaction is called decarboxylation.
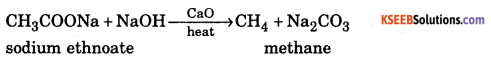
c. What is the compostion of Zeigler Natta Catalyst? ( 1 )
Answer:
Triethyl aluminium and titanium tetrachloride inert solvent.
Part – E
Answer any Three of the following questions: ( 3 × 5 = 15 )
Question 31
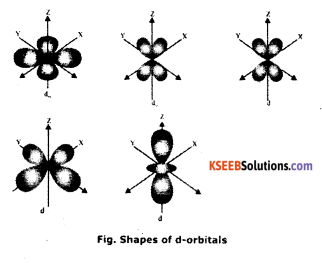
a. Draw the structure of d-orbital (Orbital whose Azimuthal quantum no = 2).
Answer:
d – orbital has 5 orientations.
b. What is Isobars ?
Answer:
Atoms of different elements having the same mass number but different atomic numbers are called isobars.
Question 32
a. Define hybridization ? Explain the hybridization in Methane molecule.
(Refer Q. No. 41)
Answer:
CH4 – Methane Molecule.
The Molecular formula of Methane is CH4
Electronic configuration of C is ground state – Is2 2s2 2p2
Elecronic configuration of C in excited state – Is2 2s1 2p3
Valance orbital representation –
The valence orbital contains unpaired electron. Hence sp3 hybridized carbon combine with 4 hydrogen atom forms methane molecule.
b. What type of orbital over lapping results in the formation of a n-bond ?
Answer:
P-P parallel / sideway / lateral overlapping.
c. Between H2S & H2O, H2O) is more polar. Why ? 3 + 1 + 1
Answer:
Because Oxygen is more electronegative than sulphur.
![]()
Question 33
a. Explain the steps involved in Born Haber cycle for the formation of NaCl.
Answer:
Step 1 : Sublimation of metallic sodium of gaseous sodium atom with enthalpy of sublimation = ∆Hs.
![]()
Step – 2 : Dissociation of molecule of chlorine to chlorine atoms with enthalpy of dissociation = ∆Hd
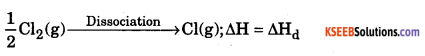
Step – 3 : Ionization of gaseous sodium with enthalpy of ionization = ∆Hi
![]()
Step – 4 : Addition of electron to gaseous chlorine atom with enthalpy of electron affinity = ∆HEa
![]()
Step – 5 : Close packing of gaseous sodium ion and chloride’ ion to form lattice structure of NaCl, with lattice chloride ion to form lattice structure of NaCl, with Lattice energy = U ;
NaCl+(g) + Cl–(g) → NaCl∆; ∆H = U
Step – 6 : But sum of all the energies will be equal to the heat of formation of one mole of sodium chloride form its reluctant i.e., ∆Hf
Na(s) + 1/2 Cl2(g) → NaCl(s); ∆H = ∆Hf
b. Define the term Entropy. What happens to Entropy when ice melts to liquid water ? ( 3 + 2 )
Answer:
It is disorderness of the system. Entropy increases.
Question 34
a. Balance MnO2 + HCl → MnCl2 + Cl2 + H2O
Answer:
Step – 1:
Assign oxidation number :
![]()
Step – 2:
Write the oxidation number changes.
Change in oxidation number of Mn + 4 to + 2 = 2
Change in oxidation number of Cl – 1 to 0 = +1
Step – 3: Cross multiply the numbers. Co-efficient 2 is multiplied to HCl and 1 is multiplied to MnO2.
MnO2 + 2HCl → MnCl2 + H2O + Cl2
Step – 4: Check the oxidation number, and 2 molecules of HCl on left hand side to balance the chlorine atoms of MnCl2. In order to balance oxygen and hydrogen atom 2 molecule of H2O has to be added on the right hand side.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
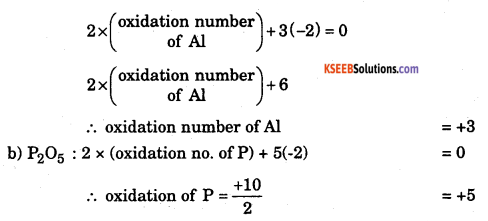
b. Calculate the oxidation number of the element underline in each of the following cases, (a) Al in Al2O3 (b) P in P2O5
Answer:
The rule used here is that the algebraic sum of the oxidation numbers of all the atoms a molecule is zero.
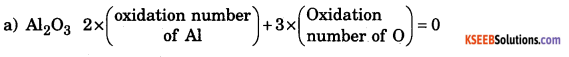
![]()
Question 35
a. Describe with a neat diagram the estimation of carbon and hydrogen by Leibig’s method.
Answer:
Principle : A known mass of an organic compound is strongly heated with dry cupric oxide (CuO), when carbon and hydrogen are quantitatively oxidized to CO2 and H2O respectively. The masses of CO2 and H2O thus formed are determined. From this, the percentages of carbon and hydrogen can be calculated.
Procedure : Pure and dry oxygen is passed through the entire assembly of the apparatus (Fig) till the CO2 and moisture is completely removed.
sample in platinum boat
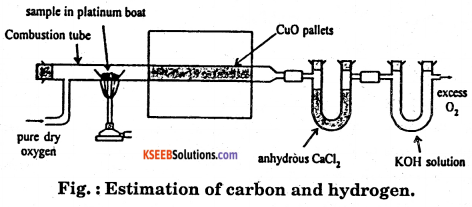
A boat containing weighed organic substances is introduced inside from one end of the combustion tube by opening it for a while. The tube is now strongly heated till the whole of the organic compound is burnt up. The flow of oxygen is continued to drive CO2 and water vapours completely to the U-tubes. The apparatus is cooled and the U-tubes are weighed separately.
Observed and Calculations.
- Mass of organic compound taken = w.g.
- Mass of water produced = x g (Increase in mass of CaCk tube)
- Mass of carbon dioxide produced = y g. (Increase in mass of KOH tube)
To determine % of carbon :
Molar mass of CO2 = 44g mol-1
Now, 44g of CO2 = contains 12 g of C.
∴ y g of CO2 will contain of \(\frac{12 y}{44}\)g of C.
This amount of carbon was present in w. g. of the substance
∴ % C = \(\frac{12 y}{44} \times \frac{100}{w}\)
To determine % of Hydrogen
Molar mass of water = 18 g mol-1
Now, 18g of H2O contains 2 g of H2
∴ x g of H2O will contain \(\frac{2 x}{18} g\) of H2
This amount of hydrogen was present in weight of substance.
∴ % H2 = \(\frac{2 x}{18} \times \frac{100}{w}\)
b. Write the functional grop for i) Aldehyde ii) Ketone. ( 4 + 1 )
Answer:
i) Aldehyde : (-CHO)
ii) Ketone : (- CO -)
Karnataka 1st PUC Chemistry Model Question Paper 2 with Answers
Time: 3.15 Hours
Max Marks: 70
Instruction:
- The questions paper has five parts A, B, C, D and E. All parts are compulsory.
- Write balanced chemical equations and draw labeled diagram wherever allowed.
- Use log tables and simple calculations f necessary (use of scientific calculations is not allowed).
Part – A
Note : Answer any nine questions: ( 9 × 1 = 9 )
Question 1.
Write the value of Avagadro’s number.
Answer:
6.023 × 1023
Question 2.
Name the fundamental particles in an atom.
Answer:
Electron, Proton, Neutrons.
Question 3.
Write the general electronic configuration of transition elements.
Answer:
(Noble Gas) (n – 1)d1–10 ns1–2
![]()
Question 4.
State Law of Conservation of Energy of mass.
Answer:
The total mass of the products in a reaction is equal to mass of the reactants ‘or’ the mass neither be created nor be destroyed.
Question5.
When is ∆H=∆u inareaction?
Answer:
H2(g) + I2(g) ⇌ 2HIg
Question 6.
Define solubility product of weak electrolyte.
Answer:
Solubility product of weak electrolyte is defined as the product of molar concentration of ions of electrolyte raised to appropriate power within saturated solution of that electrolyte.
Question 7.
What is the valency of hydrogen in hydride ion?
Answer:
– 1
Question 8.
Name the isotope of hydrogen which used In nuclear reactor.
Answer:
\(_{1}^{2} \mathrm{H}\) (deuterium)
Question 9.
Name any four alkali metals.
Answer:
Lithium, Sodium, Potassium and Rubedium.
![]()
Question 10.
What is the self linking property of an atom known as?
Answer:
Catenation.
Question 11.
Write the IUPAC name of
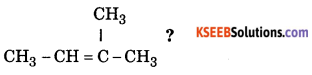
Answer:
2 Methyl 2 Butene.
Part – B
Note : Answer any Five questions: ( 5 × 2 = 10 )
Question 12.
Calculate the wave number and wavelength of the first spectral line of Lyman series of hydrogen spectrum.
Rydberg constant R = 10.97 x 106 m–1
Answer:
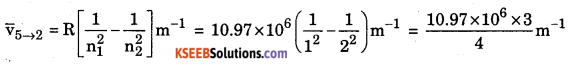
8.2275 × 106 m–1 Wave number v̄ = 8.2275 × 106 m–1
Wavelength λ = \(\frac{1}{8.2275 \times 10^{6}}\)m = 121.5 × 10–9 m = 121.5 nm
Question 13.
What is electro negativity? How does it change in a period as well as in a group?
Answer:
The ability of an atom to attract the shared electron pair (of a covalent bond) in a molecule towards itself is called electro negativity. In a period from left to right the electro negativity increases. Down a group electro negativity value decreases.
![]()
Question 14.
Mention any two characteristics of ionic compounds.
Answer:
- They have high Melting point.
- Many of it are solid in nature.
- Does not conduct in solid state but good conductors in fused or aqueous state.
- Soluble in polar solvent & insoluble in non-polar solvent.
- Ionic bond is non-directional.
- They do not exhibit Isomerism.
Question 15.
Write the acidity and calculate equivalent weight of following base.
a) Ca(OH)2 b) Fe(OH)3
Answer:
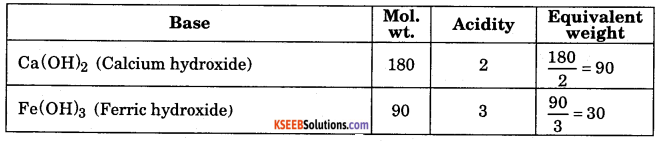
Question 16.
Among NH3, H2O and HF, which would you except to have highest magnitude of hydrogen bonding and why ?
Answer:
HF, because Flourine is more electronegative.
Question 17.
Calculate the [OH–] of a solution whose pH is 9.62.
Answer:
pH + pOH = 14 pOH = 14 – pH = 14 – 9.62 = 4.38
pOH = 4.38 = – log10 [OH– ]; 4.38 = – log10 [OH– ]
log10[ OH– ] = – 4.38 ; [OH–] = antilog (–4.38) = antilog (5 – 4.38 – 5)
= antilog (0.62 – 5) = antilog (0.62) × 10–5 = 4.169 × 10–5 mol dm–3.
![]()
Question 18.
Name the oxides of nitrogen. What are the sources ?
Answer:
Nitrogen monoxide (NO), Nitrogen dioxide (NO2), Dinitrogen trioxide (N2O3),
Dinitrogen tetroxide (N2O4), Dinitrogen pentoxide (N2O5)
The source of oxides of nitrogen are combustion of fossil fuel, especially, petroleum. They are also formed by reaction of N2 and O2 in presence of lightening.
Part – C
Note : Answer any Four questions: ( 4 × 4 = 16 )
Question 19.
4g of copper chloride on analysis was founded to contain 1.890 g of copper (Cu) and 2.110 g of chlorine (Cl). What is the empirical formula of copper chloride? [At. mass of Cu = 63.5 u, Cl = 35.5 u].
Answer:
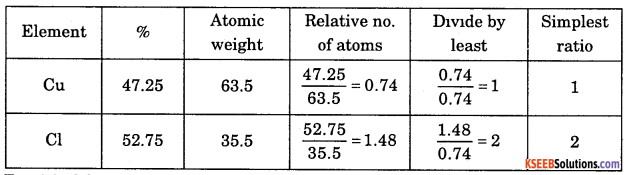
Empirical formula = CuCl2.
Question 20.
Based on VSEPR theory explain the structure of water.
Answer:
Electronic configuration of O: Is2 2s2 2p4Valence orbited representation :
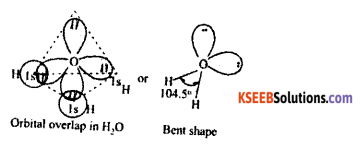
- When 2s2 orbital hybridised with 2px, 2py and 2pz forms the following sp3 hybridisation structure.
- In 2s2 and 2px 2 the electrons are paired up hence they are not involved in bond formation called loan paired of electron (L.P.)
- When sp3 hybridised O combines with 2 hydrogen atom forms Bond structure water.
Question 21.
State and illustrate Hess’s law.
Answer:
Hess’s law : Whether a chemical reaction takes place in a single step or in several steps, the total change in enthalpy remains the same.
Consider the formation of CO2 from C.
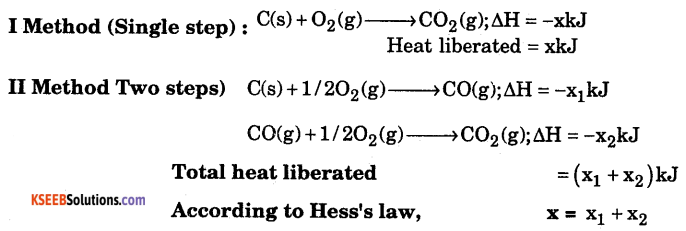
Question 22
a. Define oxidation and reduction according to electronic concept.
Answer:
Oxidation is a process in which loss of electrons take place. Reduction is a process in which gain of electrons takes place.
b. Define cathode and anode.
Answer:
Cathode is electrode towards which cations are attracted. Anode is electrode which attracts anions.
![]()
Question 23
a. Give the IUPAC name of the following compounds.
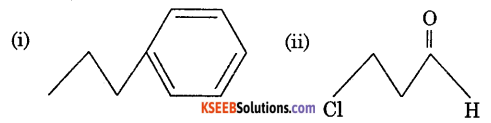
Answer:
(i) Propyle Benzene
(ii) 3 Chloro Propenal
b. In Leibig’s method. 0.24 g of organic compound on combustion with dry oxygen produced of 0.62 g of CO2 and 0.11 g of H2O. Determine the percentage composition of the compound.
Answer:
Mass of organic compound = m = 0.24 g
Mass of carbon dioxide formed = 0.62 g
Mass of water formed = 0.11 g
Percentage of carbon = \(\frac{12}{4} \times \frac{0.62}{0.24} \times 100\) = 70.45
Percentage of hydrogen = \(\frac{2}{4} \times \frac{0.11}{0.24} \times 100\) = 5.09
Percentage of oxygen = [100 – (70.5 + 5.0)] = 24.46
Question 24
a. Why does benzene undergo electrophilic substitution easily and nucleophilic substitution with difficulty?
Answer:
Because of de-localisation of 671 electrons in the benzene ring.
b. Write the balanced chemical equations for the combination of the following hydrocarbons 2.
(i) Butane (ii) Toulene
Answer:
(i) C4H10 + \(\frac{3}{2}\)O2 → 5H2O + 4CO2
(ii) C6H5CH3 + 9O2 → 7CO2 + 4H2O
Part – D
Note : Answer any Four questions: ( 2 × 5 = 10 )
Question 25
a. Summarize the Bohr’s Model of an atom.
Answer:
Bohr’s Model of an atom, the postulates are
- Electrons revolve around the nucleus of an atom in a certain definite path called Orbit or stationary state of shell.
- The shells are having different energy levels denoted as K, L, M, N
- As long as the electron remains in an orbit, they neither absorb nor emit energy.
- The electron can move only in that orbit in which angular momentum is quantized, i.e., the angular momentum of the electron is an integral multiple of \(\frac{\mathrm{h}}{2 \pi}\).
b. Mention the Merits of Bohr’s theory.
Answer:
- Explains the formation of hydrogen spectrums
- It explains the stability of atom
- Ryd berg constant is calculated by it.
![]()
Question 26
a. Explain the structure of CH4 on the basis of VSEPR theory.
b. Define electronegativity.
Answer:
The ability of an atom to attract the shred electron pair (of a covalent bond) in a molecule towards itself is called electro negativity. In a period from left to right the electronegativity increases. Down a group electronegativity value decreases.
Question 27
a. State First law of thermodynamics and write its mathematical form.
Answer:
The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. In equation form, the first law of thermodynamics is ΔU = Q − W.
b. 1 m3 of C3H4 at STP is burnt in oxygen, according to the thermochemical reaction : C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l); ΔH = – 1410 kJ mol–1 Assuming 70% efficiency, determine how much of useful heat is evolved in the reaction.
Answer:
22.4 L of C2H4 at STP produces 1410 kJ of energy.
1000 L of C2H4 at STP produces \(\frac{1410}{22.4}\) × 1000 (efficiency)
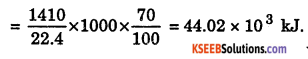
Question 28
a. Explain Arrhenius concept of acids and bases with example.
Answer:
An electrolyte which when dissolved in water, produces H ion is called acid.
An electrolyte which when dissolved in water, produces OH– ion is called base.
b. Explain the buffer of acidic buffer.
Answer:
Consider acidic buffer mixture,
CH3COOH ⇌ CH3COO– + H+ ; CH3COONa → CH3COO– + Na+
Here, acetic acid is a weak electrolyte, in it’s solution there exists equilibrium between it’s ions and molecules. Where as sodium acetate completely dissociates into it’s ions.
Therefore, the buffer mixture contains large number of CH3COO– ions followed by Na+, H+, CH3COOH.
Case i) When an acid added to this solution :
H+ ion of the acid combines with CH3COO– ion in buffer solution and makes equillibrium with acetic acid.
CH3COO– + H+ ⇌ CH3COOH
As a result pH remains constant.
Case ii) When a base is added to this solution :
OH– ion of the base combines with H+ ion in buffer solution forms water molecule.
H++ OH– → H2O.
As a result pH remains constant.
b. The pOH of a solution is 5.725. Calculate the [H+].
Answer:
pH + pOH = 14 pH = 14 – pOH = 14 – 5.725 = 8.275
pH = –log10 [H+] ⇒ 8.275 = log10 [H+] ⇒
[H+] = antilog (- 8.275) = antilog(9 – 8.275 – 9).
[H+] = antilog (0.275 – 9) = 5.309 × 10-9 mol dm-3.
![]()
Question 29
a. Describe an experiment to determine the percentage of nitrogen in an organic compound by Kjeldahl’s method.
Answer:
Principle : When a nitrogenous organic compound is heated with cone. H2SO4 using (CUSO4K2SO4) as a catalyst, the nitrogen from the compound is quantitatively converted to ammonium sulphate.
This ammonium sulphate is decomposed by heating with excess of alkali and the ammonia evolved is absorbed in known excess of a standard solution of H2SO4. Part of acid is neutralized by ammonia. The excess of acid left behind after neutralization with ammonia is estimated by back titration with standard alkali. From this, the amount of acid actually consumed by ammonia can be obtained which can be used to determine the percentage of nitrogen in the compound.
Procedure : A known exact mass of organic compound (about 0.5g) ix mixed with 10g K2SO4, Ig CuSO4 and 25 ml of conc. H2SO4. The mixture is heated strongly in a Kjeldahl’s flask. Till the contents become clear. This step is known as digestion.
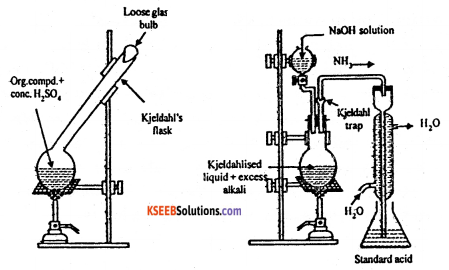
The Kjeldahl’s flask is now cooled and the liquid is heated in a round-bottomed flask with excess of caustic soda solution The ammonia evolved is absorbed in a known volume of a standard acid.
The amount of unreacted acid is determined by titrating it against a standard alkali. (NH4 )2 SO4 + 2NaOH → 2NH3 ↑+Na2SO4 + 2H2O
Observation :
- Mass of organic compound taken = Weight
- Normality of standard acid = N
- Volume of standard acid taken = V1 ml
- Volume of alkali (Normality = N) required for back titration = V2 ml
Calculation: Volume of acid used up by ammonia = Volume of ammonia produced = (V2 – V1) = V ml of normality N.
Now,
1000 ml of 1 normal NH3 = 17g NH3 = 14g N2
∴ V ml of N – normal ammonia will contain \(\frac{14 \times \mathrm{N} \times \mathrm{V}}{1000}\)gN2
This amount of nitrogen was present in w g of the compound
∴ %N2 = \(\frac{14 \times N \times V}{1000} \times \frac{100}{w}\) or %N2 = \(\frac{1.4 \mathrm{NV}}{\mathrm{w}}\)
Where, N and V are the normality and volume respectively of the acid used up by ammonia.
b. What are isomorphous salts ? Give two examples.
Answer:
Isomorphous salts are those which have same crystalline structure , e.g., MgSO4, 7H2O and ZnSO4, 7H2O are isomorphous.
![]()
Question 30
a. Discuss the molecular orbital theory of benzene.
Answer:
The electronic configuration of carbon in the ground state is
![]() ’
’
In the excited state of carbon, one of the 2s electrons gets promoted to 2p orbital. So the electronic configuration of carbon in the excited state is
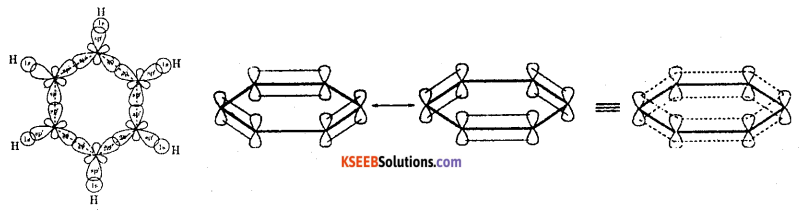
- All the six carbon atoms in benzene undergoes sp2 hybridization to form three sp2 hybrid orbitals.
- Each carbon atom in benzene possesses an unhybridised p-orbital containing one electron. They overlap sideways forming three π bonds. The π electrons are delocalised.
- Benzene is a planar molecule with a π electron cloud above and below the ring as shown in above structure. The bond angles are 120°.
- Bond length is 0.139 nm and dipole moment is zero.
- It has twelve a bonds and three π bonds.
- Two carbon atoms to form two sigma bonds and one hydrogen atom to form another sigma bonds.
Part – E
Answer any Three of the following questions: ( 3 × 5 = 15 )
Question 31
a. Calculate the mass of hydrochloric acid in 200 cm3 of 0.2 N solution of it.
What volume of this acid solution will react exactly with 25 cm3 of 0.14 N solution of sodium hydroxide?
Answer:
Normality of hydrochloric acid = 0.2;
Eq. mass of hydrochloric acid = 36.5
Mass of hydrochloric acid in one dm3 of the solution is given by
W = N × E = 0.2 × 36.5 g
∴ mass of hydrochloric acid in 200 cm3 of the solution = \(\frac{0.2 \times 36.5}{5}\) = 1.46 g
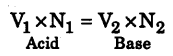
Substituting we have V1 × 0.2 = 25 × 0.14
∴V1 = \(\frac{25 \times 0.14}{0.2}\) = 17.5 cm3
b. Write the difference between isotope & isobars.
Answer:
| Isotopes | Isobars |
| Different atoms of the same elements having same atomic number but different mass number are called Isotopes. Ex: 1H1 (Protium), 1H2 (Deuterium), 1H3 (Tritium) 17Cl35 , 17CS37 |
Element having different atomic number but same mass number are called Isobars. 18Ar40 , 19K40 , 20Ca40 |
![]()
Question 32
a. 50 ml of oxygen were collected at 10° C under 750 mm pressure. Calculate volume at STP.
Answer:
V1 = 50 ml V = ?
P1 = 750 mm P2 = 760 mm
T1 = 10 + 273=283K T2 = 273.15K
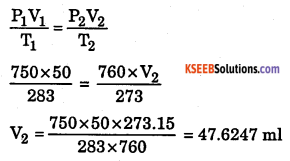
b. Give any two differences between ideal and real gas.
Answer:
| Ideal Gas | Real Gas |
| 1. Obeys ideal gas equation PV = nRT | 1. Does not obey the ideal gas equation but obeys Van der walls equation. |
| 2. Ideal gas does not exist in nature. | 2. All gases which exist in nature are real gases. |
Question 33
a. A buffer solution of pH 8.3 is prepared from ammonium chloride and ammonium hydroxide. Dissociation constant of ammonium hydroxide is 1.8 × 105. What is the mole proportion of ammonium chloride and ammonium hydroxide?
Answer:
pOH= 14 – pH = 14 – 8.3 = 5.7
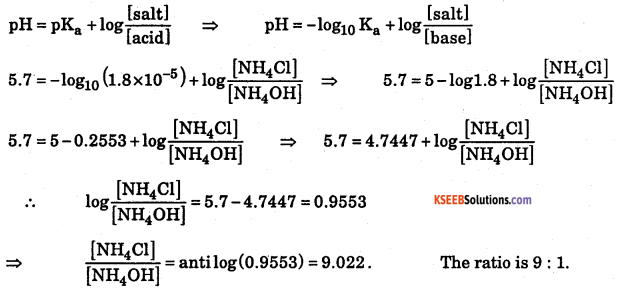
b. Write the conjugate acid for the following.
(i) C2H5NH2 (ii) CH3COO–
Answer:
(i) C6H5NH3+ (ii) CH3COOH
![]()
Question 34
a. Describe in brief the manufacture of caustic soda using the Castner-Kellner cell.
Answer:
The Castner-Kellner cell consists of large rectangular trough divided into three compartments with partition short of reaching the bottom of the tank Thus mercury in one compartment can flow into another but solution cannot mix. Graphite anodes are used in outer compartments filled with NaCl solution. The middle compartment contains very dilute solution of caustic soda and filled with iron rods as cathode.
On passing electric current Cl2 is liberated in outer compartments and sodium liberated at cathode. Mercury forms amalgam which is passed into middle compartment in which mercury acts as anode (having induced +ve potential).
At anode Na+ + e– → Na
At cathode Na + Hg → Na – Hg
2 (Na – Hg) + 2H2O → 2NaOH + Hg + H2
The concentration of NaOH goes on increasing in the middle compartment. When the concentration of NaOH reaches 20% the solution is replaced by dilute solution.
b. Give reason for diagonal relationship of lithium with magnesium.
Answer:
Both Lithium and magnesium have small size and high charge density. The electro negativities of Li is 1.0 and Mg is 1.2. They are low and almost same. Their ionic radii are similar. Hence they show similarities which is known as diagonal relationship between first element of a group with the second element in the next higher group.
![]()
Question 35
a. Describe the following in brief:
(i) Ozone depletion over Antarctica (do not write reaction)
(ii) BOD and COD
(iii) Eutrophication
Answer:
(i) Ozone depletion over Antarctica : In summer season, nitrogen dioxide and methane react with chlorine monoxide preventing ozone depletion whereas in winter, special type of clouds called polar stratosphereic could s are formed over Antartica. These clouds provide surface on which chlorine nitrate molecules formed gets hydrolysed to form HOCl. It also reacts with HCls to give Cl2. When sunlight returns to Antarctica again ozone depletion starts by free radicals.
(ii) BOD : It is amount of oxygen required by bacteria to decompose organize wastes present in water.
COD : It is the amount of oxygen (in ppm) required to oxidize the contaminants. COD is determined by using chemical oxidizing agent K2Cr2O7.
(iii) Eutrophication : The process in which nutrient enriched water bodies support a dense plant polulation which kills animal life by depriving it of oxygen and results in subsequent loss of biodiversity is known as Eutrophication.
b. Distinguish between photochemical smog and classical smog.
Answer:
| Photochemical smog | Classical smog |
| 1. It is formed by oxides of nitrogen, hydrocarbons, etc., | 1. It is formed by oxides of sulphur, carbon and particulate matter from combustion. |
| 2. It is oxidizing in nature. | 2. It is reducing in nature. |
| 3. Photochemical smog occurs in cities having large number of vehicles. It is not harmful. | 3. It occurs in both urban and rural areas. It is harmful. |
Karnataka 1st PUC Chemistry Model Question Paper 3 with Answers
Time: 3.15 Hours
Max Marks: 70
Instructions:
- The questions paper has four parts A, B, C and D.
- In part A each question carries ONE Marks. In part B, each question carries TWO marks, in part C each question carries FIVE marks. In Part D-1 carries TEN Marks and each question in D-2 carries FIVE marks
- Write balanced chemical equations and draw diagram wherever necessary.
Part – A
I. Answer of all the following questions: (1 × 9 = 9)
Question 1.
State “Law of definite proportions”.
Answer:
The given compound always contains exactly the same proportion of elements by weight.
Question 2.
Name the fundamental particle of an atom that has highest value for its e/m
Answer:
Electron.
Question 3.
Write the two resonance (canonical/contributing) structures of Ozone.
Answer:
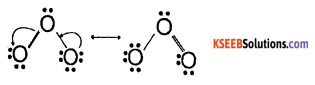
Question 4.
A molecule XY4 has four bond pairs and 2 lone pairs of electrons for its central atom. Predict the shape of the molecule.
Answer:
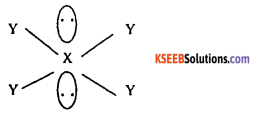
![]()
Question 5.
How many valence electrons are present around phosphorous in PCls?
Answer:
P – 15 = 1s2 2s2 2p6 / 3s2 3p3.

Question 6.
What is the change in internal energy of a system, if 10J of heat is supplied to it and 15J of work is done by it?
Answer:
Since it is a closed system.
∆u = q – w
∆u = 10J – 15J ∆u= -5J
Question 7.
H– is a Lewis base. Give reason.
Answer:
H– can donate a pair of electron. Hence it is a lewis base.
Question 8.
What is the composition of water gas ?
Answer:
CO(g) + H2(g) or a mixture of carbon monoxide and dihydrogen gas is called as water gas.
![]()
Question 9.
Which alkali metal is the strongest reducing agent ?
Answer:
Lithium of Li.
Question 10.
Draw the staggered conformation of Ethane.
Answer:
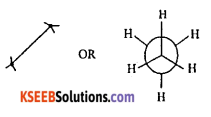
Question 11.
Mention one use of Chromatography.
Answer:
Chromatography is used to:
- Separate mixtures into their components
- purify components
- test the purity of compounds
Part – B
II. Answer any Five questions: ( 5 × 2 = 10 )
Question 12.
(i) How many significant figures are in 0.2500 g?
(ii) If the mass of one molecule of water is 18 amu, what is the mass of one mole of water molecules ?
Answer:
(i) Four
(ii) 18g.
![]()
Question 13.
(i) How does the atomic radius very down a group in the periodic table ?
(ii) Arrange the following in the decreasing order of their ionic radius : N-3, Mg+2, Na+1, O-2
Answer:
(i) Atomic radii increases down a group in the periodic table.
(ii) N-3 > O-2 > Na+ > Mg2+
Question 14.
Among N, Cu, Rn and U, identify the element that: (i) belongs to d-block, (ii) is an actionid
Answer:
(i) Cu,
(ii) U
Question 15.
(i) State Charles’ Law.
Answer:
Charle’s law states that volume of fixed mass of gas is directly proportional to absolute temperature at constant pressure.
\(\left(\frac{\mathrm{V}_{1}}{\mathrm{T}_{1}}=\frac{\mathrm{V}_{2}}{\mathrm{T}_{2}}\right)_{\mathrm{P}}\)
(ii) Give the relationship between molecular mass and density of a gas.
Answer:
(ii) d = \(\frac{P M}{R T}\)
Question 16.
What happens when (i) Sodium hydride is treated with water ?
(ii) Hydrogen peroxide is treated with lead sulphide ?
Answer:
(i) Sodium hydride when treated with water liberate dihydrogen gas
NaH(s) + H2O(I) → NaOH(l) + H2(g)
(ii) Hydrogen peroxide on reacting with lead sulphide produces lead sulphate.
4H2O2 + PbS → PbSO4 + 4H2O
Question 17.
What is the repeating unit in Organo silicon polymer? Name the starting (raw) material used in the manufacture of Organo silicon polymer.
Answer:
R2 SiO. The starting material is alyl or aryl substituted silicon chlorides.
![]()
Question 18.
How is Ozone layer formed in the stratosphere? Name a chief chemical that causes its depletion.
Answer:
In stratosphere the free oxygen atoms combine with the molecular oxygen is presence of UV radiations to form ozone.
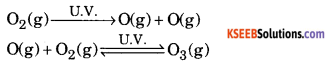
The chemical that causes its depletion is chlorofluorocarbon compounds or CFCs or fireons.
Part – C
I. Answer any Four questions: (4 × 4 = 16)
Question 19.
(i) State Heisenberg’s uncertainty principle. Give its mathematical equation.
Answer:
It is impossible to determine both the momentum (particle nature) and position (wave nature) of a moving sub atomic particle simultaneously with absolute accuracy.
Mathematically ∆x × ∆p = h / 4π where ∆x = uncertainty in position:
∆p = uncertainty in momentum ; h = Plank’s constant = 6.626 × 1O-34 Js.
(ii) Calculate the wave number of the spectral line of shortest wave length appearing in Given, R = 1.09 × 107 m1
Answer:
\(\overline{\mathbf{v}}=\mathrm{R}\left(\frac{1}{\mathrm{n}_{1}^{2}}-\frac{1}{\mathrm{n}_{2}^{2}}\right) \mathrm{m}^{-1}\)
v̄ = 1.09 × 107 m-1 \(\left(\frac{1}{2^{2}}-\frac{1}{0}\right)\)
v̄ = 1.09 × 107 (\(\frac{1}{4}\))
v̄ = 0.2725 × 107 v̄ = 2.725 × 106 m-1
![]()
Question 20.
(i) Mention two conditions for the linear combination of atomic orbitals.
(ii) Write the electronic configuration of C2 molecular. What is its magnetic property ?
Answer:
(i) (a) The combining atomic orbitals must have the same or nearly the same energy
(b) The combining atomic orbitals must have the same symmetry about the molecular axis.
(c) The combining atomic orbitals must overlap to the maximum extent.
It is diamagnetic. E.C. of C2 molecule is \(\sigma 1 \mathrm{s}^{2} \sigma^{*} 1 \mathrm{s}^{2} \sigma 2 \mathrm{s}^{2} \sigma^{*} 2 \mathrm{s}^{2} \pi 2 \mathrm{px}^{2}=\pi 2 \mathrm{py}^{2}\)
Question 21.
(i) Define “Standard Enthalpy of Vapourisation”
(ii) Write thermo chemical equation for vaporization of Ethanol (C2H3OH)
(iii) Calculate the enthalpy of vapourisation of ethanol, given enthalpies of formation of liquid Ethanol and gaseous Ethanol as -277.6 kJ and -235.4kJ respectively.
Answer:
(i) Amount of heat required to vaporize one mole of a liquid at constant temperature under standard pressure (1 bar) is called standard enthalpy of vaporization.
(ii) C2H3OH(l) → C2H3OH(g) ; ∆H = +QkJ
(iii) ΔHvap= Hp-Hr .
ΔHvap =-235.4kJ-(-277.6kJ)
ΔHvap = 42.2kJ
Question 22.
(a) For ![]()
(i) What is the oxidation number of Oxygen in (2) ?
(ii) What type of Redox reaction is it?
(b) Balance the Redox reaction using oxidation number method.
SO2 + H2S → S + H2O
Answer:
(i) -2
(ii) Disproportionation or Disproportionate reaction.
(b)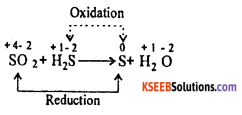
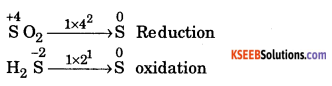
Multiply oxidation equation by 2 and reduction equation by 1.
SO2 → S
2H2S → 2S
Add both the equations
SO2 + 2H2S → 3S
Balancing the atoms of hydrogen and oxygen by introducing suitable number of H2O molecules to the product.
![]()
Question 23.
(a) How is sulphur detected using the ‘Sodium fusion extract’ of the given organic compound ?
(b) Give the IUPAC name of
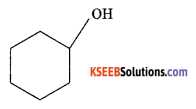
Write the bond line diagrame of 2-methyl pentane.
Answer:
(a) Te sodium fusion extract is acidified with acetic acid and lead acetate. A black precipitate of lead sulphite is formed which indicates the presence of sulphur.

(b) Cyclohexanol
Question 24.
(a) Give equation for each of the following reactions.
(i) Water is dropped on Calcium carbide
(ii) Hydrogen bromide is added to propene in presence of peroxide
(iii) Phenol is heated with Zinc dust.
(iv) Benzene is treated with Chlorine in presence of Ferric chloride.
Answer:
(a) (i) CaC2 + 2H2O → Ca(OH)2 + C2H2
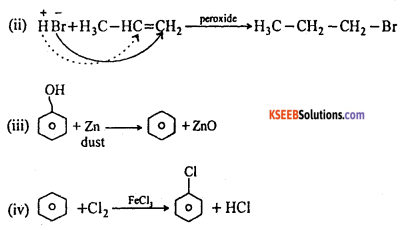
(b) (i) Write the equation for the steps involved in the mechanism of nitration of Benzene between Toluene and Nitrobenzene which one is more reactive towards Nitration ?
Answer:
Nitration benzene reacts with a mixture of concentrated nitric acid and concentrated sulphuric acid at 50°C to form nitrobenzene.
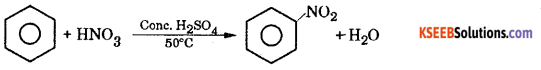
Mechanism: This involves the following steps
Step 1: Generation of electrophile nitronium ion NO2+
HNO3 + 2H2SO4 → NO2+ + H3O++ 2HSO4–
Step 2: The electrophile NO2+ attacks the benzene ring to form a carbocation which is resonance stabilized.
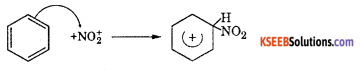
Step 3: Loss of proton to give nitrobenzene. The proton is removed by HSO4–
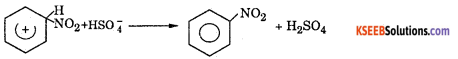
Part – D
Answer any Four questions: ( 4 × 5 = 20 )
Question 25
a. Calculate the mas of Magnesium required to completely react with 250 cm3 of O.1 M HCl.
Given : Mg + 2HCl – MgCl2 + H2 Atomic mass of Mg = 24
Or (Internal choice)
100cm3 of a solution of HCl completely neutralizes 25cm3 of 0.1 M NaOH.
Calculate the mass of HCl present in 100cm3.
(b) Mention two postulates of ‘Dalton’s atomic theory’.
Answer:
(a) 250 × 0.1 M = 25 millimoles of HCl
From the equation it is clear that 2HCl is reacting with one Mg.
∴ 25 millimoles of HCl rect with 12.5 millimoles of Mg
∴ Mass of magnesium required = volume of Mg × atomic weight = 12.5 × lO-3 × 24 = 0.3 g of Mg.
OR
(a) VHcl = 100cm3, VNaOH = 25cm3, MHCl = ?, MNaOH = 0.1M
aHcl VHcl × MHcl = VNaOH × MNaOH × aNaOH
here aHCl = basicity of HCl = 1, aNaOH = acidity of NaOH = 1
MHcl = \(\frac{25 \times 0.1}{100}\) = 0.025m
Mass of HCl present in 100cm3
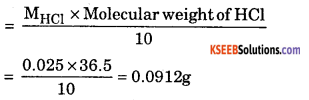
b. Mention two postulates of ‘Dalton’s Atomic theory’
Answer:
The postulates of Dalton’s atomic theory are :
- The matter consists of indivisible atoms.
- All the atoms of a given elements have identical properties including identical mass. Atoms of different element differ in mass.
- Compounds are formed when atoms of different elements combine in a fixed ratio.
OR (Internal choice)
![]()
What is Empirical formula ? Give an example for a compound whose Empirical formula and molecular formula are the same
Answer:
Emperical formula represents the simplest whole number ratio of various atoms present in a compound.
Example : C2H8.
Question 26.
(a) Define the terms:
(i) Bond order
(ii) Bond length
(iii) Bond enthalpy
Answer:
(i) It is the number of covalent bonds holding the atoms in the molecule.
Example: If the bond is formed by the sharing of two electron pairs, then the bond order is 2. O=O or C = C bond in alkenes.
(ii) Bond length: It is the equilibrium distance between nuclei of two bonded atoms in a molecule.
(iii) Bond enthalpy: It is the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state.
b. With respect to the formation of Ethane molecule mention.
(i) hybridation of Carbon. (ii) number of sigma bonds in the molecule.
Answer:
(i) sp2
(ii) 5
Question 27.
(a) Write three postulates of ‘Kinetic theory of gases’.
Answer:
- Gases are made up of large number of the minute particles.
- Pressure is exerted by a gas
- There is no loss of kinetic energy.
- Molecules of gas attract on one another.
- Kinetic energy of the molecule in directly proportional to absolute temperature.
- Actual volume of the gaseous molecule very small.
- Gaseous molecules are at always in motion.
- There is more influence of gravity on movement of gaseous molecule.
(b) Two gases A & B have critical temperatures as 250 and 125 K respectively. Which one of these can be liquefied easily and why ?
Answer:
Gas a can be easily liquefied. Because higher critical temperature, greater is the inter molecular force of attraction.
Question 28.
(a) Calculate the pOH of a solution obtained when 0.05 mol NH4Cl is added and dissolved in 0.025M ammonia solution. Kb for ammonia is 1.77 × 10-5
(b) For the equilibrium : BaCO3(s) ⇌ BaO(s) + CO2(g)
(i) Write the expression of Kp.
(ii) What is the effect of ‘increase in pressure’ on the above equilibrium ?
Answer:
(a) pKb = – log[Kb]
= – log [1.77 × 10-3]
= 5 – 0.2480 = 4.752
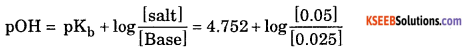
pOH= 4.752 + log2 = 4.752+ 0.3010 = 5.053
(b) (i) Kp = PCO2
(ii) Increase in pressure shifts the equilibrium to left or backward reaction is favoured.
Question 29.
(a) Give reason (i) Coodination number of Be is 4, but that of Mg is six
(ii) Lithium iodide is covalent but potassium iodide is ionic.
Answer:
(a) (i) Beryllium does not have ‘d’ subshell in n = 2 level. It has only 4 valence orbital.
Hence it can have a coordination number 4.
Mg has ‘d’ subshells in n = 3 level. Hence it has more valence orbital. Therefore it can have a coordination number 6.
(ii) Lithium iodide is covalent because of the smaller atomic size of lithium atom, it distorts to the electron to the electron cloud of iodine towards it. Or K has less polarizing power than Li+.
(b) Compared the 2nd Ionisation enthalpies and Hydration enthalpies of Alkali and Alkaline earth metals/ions.
Answer:
Alkali metals have higher value of 2nd ionization enthalpy than alkaline earth metals.
since they achieve a stable noble gas configuration after loosing 1 electron from ns1 shell.
Alkaline earth metal ions have higher value of hydration enthalpy because of their smaller ionic size than alkali metals.
(c) What is the chemical formula of plaster of paris ?
Answer:
CaSO4. 1/2H2O
![]()
Question 30
(a) Name the method by which Halogen present in an organic compound is estimated ? O.1 g of an organic compound gives 0.08g of silver bromide, Calculate the percentage of bromine.
Atomic masses : Ag = 108, Br = 80
(b) What is inductive effect ?
Which one of the following shows maximum hyper conjugation effect ?
CH3CH = CH2, (CH3)2 = C = CH2 CH2 = CH2
Answer:
(a) Carius method
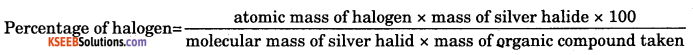
molecular mass of AgBr = 108 + 80 = 188
Mass of silver bromide = 0.09g
Mass of organic compound = O.1 g
% of bromine = \(\frac{80 \times 0.08 \times 100}{188 \times 0.1}\) = 34.04%
(b) The percentage displacement of bonded sigma electrons along the chain of carbon atoms due to the presence of a substitutent group of different electro negativity is called inductive effect.
(CH3)2 C = CH2
Part – E
Answer any three questions: (3 × 5 = 15)
Question 31.
(a) For the Element with atomic number 24:
(i) Write the electron configuration
(ii) Write the value of u & 1 for its electron in the valence shel.
(ii) How many unpaired electrons are present in it ?
Answer:
(a) (i) 1s2 2s2 2p6 3s2 3p6, 4s1 3d5
(b) n = 4, l = 0
(c) Six or 6.
(b) What is photo electric effect ? Does the effect support particle nature or wave nature of light ?
Answer:
The effect in which the electrons gets ejected when certain metals are exposed to a beam of light is called as photo electric effect.
It supports the particle nature of light.
![]()
Question 32.
(a) What is a spontaneous process ?
For the equilibrium A + 2B ⇌ C
∆H is + 400 kJ and ∆S is + 200 JK-1
Calculate the temperature above which the reaction becomes spontaneous ?
Answer:
The process that takes place on its won without the assistance of external agency is called spontaneous process.
∆G = ∆H – T∆S
At equilibrium, ∆G = 0
T∆S = ∆H
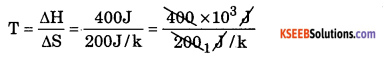
T = 2000 K.
(b) For Cl2 → 2Cl Assign the sigans for ∆G and ∆S.
Answer:
∆H = +ve (endothermic reaction)
∆S = -ve (entropy increases)
Question 33.
(a) Calculate the solubility of Ag2CrO44 in 0.1M AgNO3Ksp of Ag2 CrO4 = 1 × 10-12
Ans:
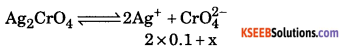
Ksp = [Ag+]2 [CrO42- ]
1 × 10-12 = (2 x 0.1)2 [CrO42- ]
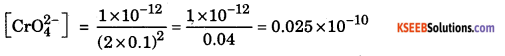
b. An aqueous solution of sodium acetate has pH greater than 7.
Explain with equation.
Answer:
Sodium acetate is a salt of a strong base and a weak acid which undergoes hydrolysis to form strong alkaline (basic) solution. Hence the pH is greater than 7.
CH3COONa + H2O ⇌ CH3COOH + NaOH
![]()
Question 34.
(a) Give reasons :
(i) The stability of +3 oxidation state of 13 group elements decreases down the group.
(ii) Boron is used as control rods in nuclear reactors.
(iii) Graphite is soft and slippery.
Answer:
(a) (i) The stability of +3 oxidation state of group 13 elements decreases down the group due to the inert pair effect on ns2 electrons. This is because of the poor shielding effect of electron in ‘d’ and ‘f’ orbitals.
(ii) Because it absorbs neutrons
(iii) Due to the weak vander waal’s force of attraction between the layers of hexagonal rings.
b. Complete the following equation :
(i) 2Al + 2 NaOH + → 6H2O
(ii)![]()
Answer:
(i) 2Al + 2NaOH + 6H2O → 2Na + [Al(OH)4 ] + 3H2
(ii) ![]()
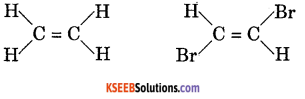
Question 35.
(a) Give two tests to distinguish between alkanes and alkenes.
(b) Naphthalene is an aromatic compound justify the statement using Huckle rule.
(c) Draw cis and trans structures of CHBr = CHBr
Answer:
(a) (i) Test with bromine water or CCl4
Alkenes react with bromine water of CCl4 to form dibromo alkane, Where as alkanes do not show such rection.
(ii) Test with alkaline. KMnO4 or Baeyer’s reagent test:
Alkenes react with Baeyer’s reagent to produce glycol, Where as alkenes do not show such reaction.
(b) According to Hucle rule, if naphthalein is an aromic compound it should possess (4n + 2) π electrons delocalized in the ring.
In naphthalein, n = 2
Number of delocalized p electrons = (4n + 2) = 4 × 2 + 2
= 10π electrons
Hence naphthalein is an aromatic compound.
(c)
Karnataka 1st PUC Chemistry Model Question Paper 4 with Answers
Time: 3.15 Hours
Max Marks: 70
Instructions:
- The questions paper has four parts A, B, C and D.
- In part A each question carries ONE Marks. In part B, each question carries TWO marks, in part C each question carries FIVE marks. In Part D-1 carries TEN Marks and each question in D-2 carries FIVE marks
- Write balanced chemical equations and draw diagram wherever necessary.
Part – A
I. Answer of all the following questions: (1 × 9 = 9)
Question 1.
What is the S.I. Unit of density ?
Answer:
Kg/m2 or kgm-3
Question 2.
How do isotopes of an element differ from one another ?
Answer:
Atoms having same atomic number but different atomic masses.
OR
Isotopes of an element differ only in the number of protons.
Question 3.
What are representative elements ?
Answer:
Elements of s-block and p-block are representative elements.
![]()
Question 4.
Between CO & CO2, which diffuses faster ?
Answer:
CO diffuses faster.
Question 5.
Give a chemical reaction for which ∆H = ∆U.
Answer:
2H(g) ⇌ H2(g) + I2(g)
Question 6.
Give the relation between Kp and Kc for the equilibrium N2(g) + 3H2(g) ↔ 2NH3(g).
Answer:
Kp = Kc = (RT)2
Question 7.
What is the chemical used in Clarke’s process to remove temporary hardness of water ?
Answer:
Lime or Ca(OH)2 or Calcium hydroxide.
Question 8.
Write the General outer electronic configuration of s-block elements.
Answer:
General electronic configuration of s-block elements is ns1-2
Question 9.
Complete the following equation 2Fe+2 + 2H+ + H2O2 → ……. + H2O
Answer:
2Fe+2+ 2H+ + H2O2 → 2Fe+3+ 2H2O
Question 10.
Suggest a suitable method to separate sugar and salt from an aqueous solution containing them.
Answer:
Fractional crystallization is a suitable method to separate sugar and salt from an aqueous solution containing them due to difference in their solubilities in water.
![]()
Question 11.
Trans-2 Butene has higher melting point than cis-2-butene, why ?
Answer:
Because more close packing of molecules is possible in trans-isomer due to more symmetry.
Part – B
II. Answer any Five questions: (5 × 2 = 10)
Question 12.
Name the quantum number that specifies
(i) Size of an orbital
(ii) Shape of an orbital.
Answer:
(i) Principal quantum number specifies size of an orbital
(ii) Azimuthal quantum number specifies shape of an orbital.
Question 13.
(i) Distinguish between Sigma and Pi bonds.
Answer:
| Sigma (σ) Bond | Pi (π) Bond |
| 1. The bond is formed by the axial overlap of the atomic orbitais. | 1. The bond is formed by the sidewise overlap of the atomic orbitais. |
| 2. s-orbitais can take part in the sigma bond formation | 2. s-orbitais do not take part in the pi-bond formation. |
Question 14.
Give the reasons for the deviation of real gases from ideal behaviour ?
Answer:
- There is no intermolecular force of attraction within gaseous molecules (at high pressure and low temperature there is appreciable intermolecular force of attraction).
- The actual volume of the molecule is negligible as compared to total volume of the gas. (At high pressure the gaseous molecule occupy minimum volume, which is not negligible).
Note: Because of this deviation Vander waal modified the ideal gas pressure and ideal gas volume respectively.
![]()
Question 15.
Why do group 1 metals have lower first ionisation Enthalpy than corresponding group 2 metals.
Answer:
- Size of 1st group metals is greater than size of 2nd group metals, hence effective nuclear charge increase of 2nd group metal.
- Upon losing one electron, 1st group metals attain stable octet structure.
Question 16.
Justify the position of Hydrogen in the periodic table.
Answer:
Hydrogen has been placed at the top of the alkali metal family.
Hydrogen and alkali metals resemble in the following aspects.
- Electronic configuration : Hydrogen has one electron in its valance shell like the alkali metals H – 1s1.
- Electropositive character : As both hydrogen and alkali metals form monovalent cations by losing the electron in the valance shell. H → H+ + e–
Question 17.
What happens when formic acid is heated with Cone. H2SO4? Give the equation.
Answer:
Dehydration takes place or CO is formed
![]()
Question 18.
What is meant by Biochemical Oxygen Demand (BOD)? What is its significants?
Answer:
The amount of oxygen required by bacteria to break down the organic matter present in a certain volume of a sample of water is called Biochemical Oxygen Demand (BOD) The amount of BOD in the water is a measure of the amount of organic material in the water, in terms of how much oxygen will be required to break it down biologically.
Part – C
I. Answer any Four questions: (4 × 4 = 16)
Question 19.
A compound contains 4.07% hydrogen, 24.27% Carbon and 71.65% Chlorine. Its molar mass is 98.96. Calculate is Empirical and Molecular formulae.
Answer:
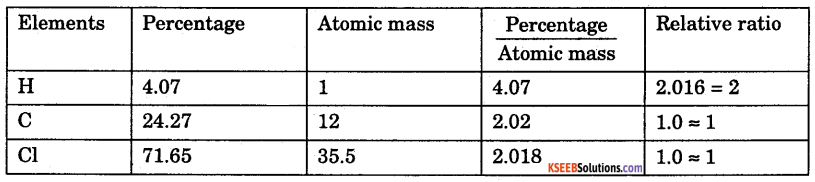
Empirical formula = CH2Cl
Molecular formula = n × Empirical formula
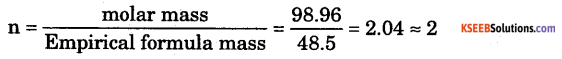
Molecular formula = 2 × CH2Cl = C2H4Cl2.
![]()
Question 20.
(a) Explain the formation of methane molecule on the basis of hybridization.
Answer:
The electronic configuration of the central carbon atom (z = 6) in methane is 2, 4. Each of the four electrons present in the valence shell forms shared pairs with the electrons of the hydrogen atoms as shown below :
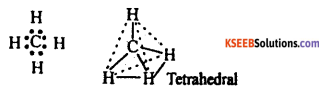
Methane has a tetrahedral structure which is multiplanar, in which carbon atom lies at the centre and the four hydrogen atoms lie at the four comers of regular tetrahedran. All H – C – H bond angles are 109.5°
(b) Between O2 & O2– , which one has higher bond order ?
Answer:
O2 has higher bond order than O2–
Question 21.
(a) What is Oxidation Number ? What is the oxidation Number of Cl is KClO3?
Answer:
Oxidation number is the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that the electrons in a co-valent bond belong entirely to the more electro negative elements.
Oxidation number of Cl in KClO3 is +5.
(b) Write separate equations for the oxidation and reduction reactions occurring in the following redox reaction.
2Fe + 2HCl → FeCl2 + H2
Answer:
2Fe + 2HCl → FeCl2 + H2
Fe → Fe2+ +2e–
2H+ + 2e– → H2
Question 22.
(a) What are Isothermal and Adiabatic process ?
Answer:
Isotherman process : A process is said to be isothermal in case the temperature of the system remains fixed or constant i.e., operation is carried at constant temperature.
Adiabatic process : In an adiabatic process, no exchange of heat between the system and the surroundings is possible i.e., the system is completely isolated or insulated from the surroundings.
(b) State the II law of Thermodynamics. Give the equation that relates Gibbs energy with Entropy and Enthalpy of a system ?
Answer:
Second law of thermodynamics can be stated in a number of forms:
- All spontaneous processes or naturally occurring processes are thermo dynamically irreversible.
- Without the help of an external agency, a spontaneous process cannot to reversed.
- The entropy of an isolated system increase if it is to be spontaneous in a particular direction.
- In a non-isolated system, the total entropy of both the system and surroundings must increase or must be positive.
- The total entropy of the universe must tend to increase in a spontaneous process.
G = H – TS
![]()
Question 23.
(a) Giving justification, catogorise the following species as Nuclophile or Electrophile.
(i)SO3, (ii) C2H5O
Answer:
(i) SO3 – Electoplite, because it is an electron deficient and accepts a pair of electrons.
(ii) C2H2O – Nucleophile because it is an electron rich and donates electrons.
OR (Internal Choice)
Mention the differences between Fractional distillation and Steam Distillation.
Answer:
| Fractional distillation | Steam distillation |
| 1. Liquid. and impurities must process different boiling points. | 1. Liquid must be steam voilatile impurities present must be no volatice. |
| 2. Liquid may or may not be mixable with water | 2. Liquid must not mixable with water. |
(b) Write the IUPAC names of the following compounds.
Answer:
OR (Internal Choice)
Explain position isomerism with suitable example.
Answer:
The isomers differ with respect to the position of the multiple bonds, substituents or functional groups
Ex . 
Question 24.
(a) Give equations for the reaction that occurs when
(i) Ehyene is treated with Baeyer’s reagent.
(ii) Enthyene is treated with sodium metal in the ratio I : 2.
Answer:

![]()
(b) Explain Friedel – Craft’s acylation with an example.
Answer:
Friedel-Craft’s acylation : Reaction between benzene and acetyl chloride in presence of anhydrous AlCl3, is called Friedel-craft’s acylation.
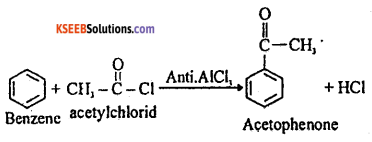
Part – D
Answer any Four questions: (4 × 5 = 20)
Question 25.
(a) Write any three postulates of Bohr’s atomic model.
Answer:
Bohr’s Model of an atom, the postulates are
- Electrons revolve around the nucleus of an atom in a certain definite path called Orbit or stationary state of shell.
- The shells are having different energy levels denoted as K, L, M, N, ……
- As long as the electron remains in an orbit, they neither absorb nor emit energy.
- The electron can move only in that orbit in which angular momentum is quantized, i.e., the angular momentum of the electron is an integral multiple of \(\frac{\mathrm{h}}{2 \pi}\).
b. Calculate the energy with 5th orbit of hydrogen atom.
Answer:
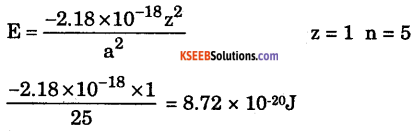
Question 26.
Define enthalpy of formation.
Calculate Enthalpy of formation of C2H6(g) given the enthalpy of combustion of C2H4(g) C2H6(g) and H2(g) as – 140 kJ/mol, -1550 kJ/mol and -286 kJ/mol respectively.
Answer:
(a) Heat of formation of a compound is the change in enthalpy produced when one mole of the compound is formed from its elements denoted as ∆Hf.
(b) C2H4(g) + CO2(g) → 2CO3(g) + 2H2O(g) ∆cH° = — 140kJ/mol … (1)
H2(g) + \(\frac{1}{2}\) O2(g) → H2O(g) ∆cH° =-286kJ/mol …(2)
C2H6(g) + \(\frac{7}{2}\) O2(g) → 2CO2(g) + 3H2O(g) ∆cH° = -1550kJ/mol … (3)
Required equation
C2H4(g) + H2(g) → C2H6(g) ∆fH° = ?
Reverse equation (3)
2CO2(g) + 3H2O(g) → C2H6(g) + \(\frac{7}{2}\) O2(g) ∆cH° = -1550kJ / mol …(4)
Add equation (1), (2) and(4)
H2(g) + \(\frac{1}{2}\) O2(g) → H2O(g)
C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(g)
2CO2(g) + 3H2O(g) → C2H6(g) + \(\frac{7}{2}\) O2(g)
C2H4(g) + H2(g) → C2H6(g)
∆fH° = 15550 kJ/mol – 140kJ/mol – 286 kJ/mil
∆fH° = 1124kJ/mol.
![]()
Question 27.
(a) (i) State Le-Chatelier’s principle,
(ii) What is common ion effect. Give an example.
Answer:
(i) Definition : “when a constraint applied to a system at equilibrium in a reversible reaction, the equilibrium shifts so as to nullify the constraint”.
[Constraint is change in temperature or pressure, addition of reactant or product]
(ii) Supression in degree of dissociation of a weak electrolyte by addition of a common ion is called common ion effect.
Example: CH3COOH and CH3COONa
OR (Internal Choice)
(i) What is the pH of 1M NaCl at 298K.
(ii) What happens to the pH of a solution of Acetic acid when some sodium acetate is dissolved in it? Explain
Answer:
(i) pH = 7
(ii) pH of the acid solution increases
(iii) Because of common ion effect dissociation of acetic acid decreased and hence [H+] decreases.
(b) Acetic acid has a dissociation constant of 1.8 × 10-5, Calculate the pH value of the decinormal solution of Acetic acid.
Answer:

pH = -log[H+] = -log 01.34 × 10-1 = 2.872
OR (Internal Choice)
Calculate the ionization constant of 0.05M weak acid if it’s degree of dissociation is 0.018.
Answer:
\(\mathrm{K}_{\mathrm{a}}=\frac{\alpha^{2}}{\mathrm{C}}=\frac{(0.018)^{2}}{0.05}=6.48 \times 10^{-3}\)
Question 28.
(a) Describe LCAO method for the formation molecular orbitals of Hydrogen. Write the energy level diagram for these orbitals.
Answer:
The formation of bonding molecular orbital when two atomic orbitals of hydrogen atoms (Is orbital) combine by the addition of their wave functions is shown below.
The atomic orbitals of these atoms may be represented by the wave function TA, TB Mathematically, the formation of molecular orbitals may be described by the linear combination of atomic orbitals that can take place by addition and by substruction of wave functions of individual atomic orbitals as shown below :
Ψmo = ΨA ± ΨB
Therefore, the two molecular orbitals σ and are σ’ formed as
σ = ΨA = ’ΨB
σ’ = ΨA = ΨB
The MO σ formed by the addition of atomic orbitals is called the bonding molecular orbital while the molecular orbital σ’ formed by the substraction of atomic orbital is known as antibonding molecular orbitals.
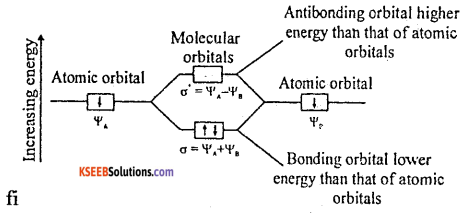
(b) Give two difference Bonding and Antibonding molecular orbitals.
Answer:
| Bonding molecular orbital | Antibonding molecular orbital |
| 1. A bonding molecular orbital is formed by the linear combination of two atomic orbitals when their wave functions are added. | 1. An antibonding molecular orbital formed by the linear combination of two atomic orbitals when their wave functions are added. |
| 2. It is formed when the lobes of the combining atomic orbitals have same sign | 2. It is formed when the lobes of the combining atomic orbitals have opposite signs. |
![]()
Question 29.
(a) What is the formulae of inorganic benzene ? Write the structure of Diborane and explain the nature of bonding in it.
Answer:
Formulae of inorganic benzene is B3N3H6.
Structure of Diborane
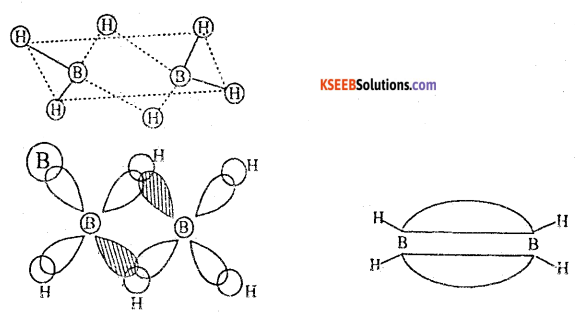
Bonding in diborane : Each B atom uses sp3 hybrides for bonding. Out of the four sp3 hybrids one each B atom, one is without electrons shown in shaded region. The terminal B – H bonds are normal 2 – centre – 2 electron bonds but the two bridge bonds are 3 – centre – 2 – electron bridge bonds are also referred to as balance bonds.
(b) What are Fullurenes ? How are they prepared ?
Answer:
Carbon exhibits many allotropic forms: both crystallins as well as amorphous. Diamond and graphite are two well known crystalline forms of carbon, third form of corbon known as fullerences.
Fullerenes are made by heating of graphite in an electric are in the presence of inert gases such as helium or argon. The sooty material formed by condensation of vapourised Ca small molecular consists of-mainly C60 with smaller quantity of C70 and traces of fallerences consiting of even number of carbon atoms upto 350 or above.
Question 30 a.
(a) Writ the principles involved in the estimation of (i) Halogens (ii) Sulphur present in an organic compound.
Answer:
(i) Halogens : Halogens reacts with silver nitrate to for precipitate of silver halides.
X + Ag+ → AgX .
X represents a halogen -Cl, -Br or -1
(ii) Sulphur
S++ [Fe(CN)5NO]2 → [Fe(CN)5NOS]4-
Sulphur reacts with nitropresside to form a violet colour complex.
(b) Name the element estimated by Kjeldahl’s method.
Answer:
Nitrogen is estimate by Kjeldahl’s method.
Part – E
Answer any Three questions: (3 × 5 = 15)
Question 31.
(a) Calculate the mole fraction of Ehtanol (C2H5OH) in the solution containing 20g of Ethanol and 100g of water.
Answer:
Weight of ethanol = 20g
Weight of water = 100g
Molecular mass of C2H5 – OH = – 46
No of moles of ethanol = \(\frac{20}{46}\) = 0.4348 moles.
n8 = No of moles of water = \(\frac{100}{18}\) = 5.555 moles
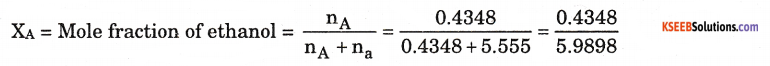
XA = 0.07259
(b) How are 2 mole NaOH and 2 Molar NaOH different ?
Answer:
2 mole NaOH
1 mole of NaOH = 40g of NaOH = 6.023 × 1023 molecules.
2 mole of NaOH 2 × 40g = 80g of NaOH
= 2 × 6.023 × 1023 molecules.
2 molar NaOH
1 molar NaOH = 40g of NaOH in 1000 ml of water
2 molar NaOH = 80g of NaOH in 100 ml of water
= 2 moles of NaOH dissolved in 1000 ml of its solution.
![]()
Question 32.
(a) Sate Gay Lussac’s law.
Calculate the pressure exerted by 4 mole of a gas occupying a volume of 1.5m3 at 100 K. Given R = 8.314 J/k/mol.
Answer:
(i) Gay Lussac’s law (Pressure – temperature relationship)
Gay Lussac’s law states that at constant volume, pressure of a fixed amount of a gas varies directly with the temperature.
(ii) Given n = 4 moles
V = 1.5m3, T = 100k, R = 8.314 J/k/mol, P = ?

(b) Draw z vs P graph for an Ideal gas, and CO2 gas
Answer:
Question 33.
(a) What are buffer solutions ? Given an example for an acidic buffer.
Answer:
The solution which resist the pH or pOH when an acid or base is added to it. Buffer solution is a mixture of weak electrolyte and its salt.
A solution containing a weak acid and its salt with a strong base.
(i) CH3COOH + CH3COONa
(ii) HCOOH + HCOOK
(b) Calculate the solubility of A2 x 3 pure water, Assuming that neither king of ion reacts with water. The solubility product of A2X3 Ksp = 1.1 × 10-23 .Explain with equation.
Answer:
ksp =[A3+]2 [X-2]3 = [2s]2 [3s]3 =108s5
S5 = \(\frac{1.1 \times 10^{-23}}{108}\) = 1 × 10-25
S5 = 1 × 10-5 mol dm-3
Question 34.
(a) Give equations for the reactions that occur when
(i) Sodium metal is dropped into water
(ii) Magnesium metal is heated in air.
(iii) Sodium peroxide dissolves in water.
Answer:

Hydrogen gas is liberated.
(ii) NO2 gas is liberated.
2Mg(NO3)2 → 2MgO + 4NO2 + O2
(iii) H2O2 is formed.
Na2O2 + 2H2O → 2NaOH + H2O2
b. What happens when limited amount of CO2 gas is passed into milk of lime ? Give equation.
Answer:
Lime water turns milky or white Ca(OH)2 + CO2 → CaCO3 + H2O
![]()
Question 35.
(a) Between -NO2 and -Br, which one of these is a in eta directing group? Write equation for the conversion of Benzene into p-Bromonitrobenzene.
Answer:
-NO2 is meta directing group.
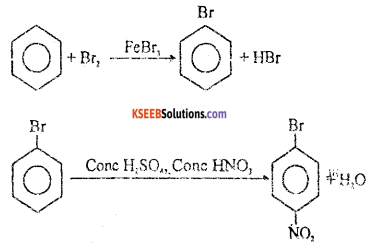
(b) State anti Merkovnikoff s rule and explain with an example.
Answer:
In the presence of peroxide, addition of HBr to un symmetrical alkenes like propene takes place contrary to the Merkovnikoff s rule. This happens only with HBr but not with HCl or HI. This reaction is know as peroxide or Kharash effect or anti- Merkovnikoff s rule
