Students can download Class 10 Science Chapter 4 Carbon and Its Compounds Important Questions, KSEEB SSLC Class 10 Science Important Questions and Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka SSLC Class 10 Science Important Questions 4 Carbon and Its Compounds
Question 1.
What is carbon? What is its chemical symbol?
Answer:
Carbon is a chemical element with atomic number 6. Its chemical symbol is C.
![]()
Question 2.
Write the structure of carbon atom.
Answer:
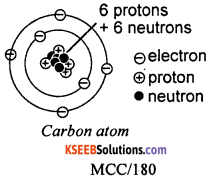
Question 3.
What product is produced when carbon is burnt?
Answer:
Carbon on burning produces carbon dioxide.
Question 4.
State the properties of carbon compounds.
Answer:
- Most carbon compounds are poor conductors of electricity.
- They have low melting and boiling points compared to ionic compounds.
- Forces of attraction between the molecules are not very strong.
- Bonding in these compounds does not give rise to any ions.
Question 5.
Explain the importance of carbon in our lives.
Answer:
Carbon is found everywhere in our lives. The food that we eat contains carbon atoms and compounds of carbon. The fuels that we use have carbon atoms in them.
The paper we use, the medicines we consume, the clothes we wear, the soaps and detergents that we use, plastics and polymers that we employ to meet our needs are all made of carbon atoms. Life on earth is itself carbon based. Carbon constitutes the most essential component of all living systems. It is impossible to think of life without carbon.
Question 6.
How is carbon found in nature?
Answer:
Carbon is present in the atmosphere in the form of carbon dioxide. Carbon is found in the earth’s crust in the form of carbonates and bicarbonates. All living systems contain carbon in one form or the other.
Question 7.
Most of the things we consume or use in our day-to-day life are made up of compounds of carbon. Explain this statement.
Answer:
If we make a list of all the things we consume or use in a day, most of the things such as soaps, detergents, newspapers, books, furniture, food items etc., are made up of compounds of carbon.
Except metal objects and ceramics, most other materials are known to contain carbon compounds. This shows that the things we consume or use are made up of compounds of carbon.
Question 8.
What is the percentage of carbon dioxide in the earth’s atmosphere? What is the percentage of carbonates and bicarbonates in the earth’s crust?
Answer:
The atmosphere of the earth contains carbon dioxide to the extent of 0.03%. Carbon is found in the earth’s crust in the form of carbonates and bicarbonates to the extent of 0.02%.
Question 9.
Show with an experiment that common sugar contains carbon.
Answer:
Take a small amount of sugar on an iron plate. Heat it strongly. We will observe a black residue as the sugar gets charred. This shows that common sugar is rich in carbon atoms. Substances that get charred on heating/buming usually contain carbon.
Question 10.
How do you confirm that carbon on burning produces carbon dioxide?
Answer:
The gas produced when carbon is burnt turns limewater milky. The gas also puts out a candle flame. This shows that the gas produced by burning of carbon is carbon dioxide.
Question 11.
What is the total number of electrons present in an atom of carbon? What is the valency of carbon?
Answer:
There are six electrons in a neutral carbon atom. Carbon is tetravalent. This means the valency of carbon is four.
![]()
Question 12.
How many valence electrons are there in an atom of carbon?
Answer:
An atom of carbon contains four valence electrons. This means that an atom of carbon has four electrons in its outermost shell.
Question 13.
What kind of chemical bonds are present in most compounds of carbon?
Answer:
Covalent bonds are present in most compounds of carbon.
Question 14.
Mention three special characteristics of the carbon atom that are responsible for its innumerable compounds.
Answer:
The following are the reasons for innumerable compounds of carbon:
- The tetravalency of carbon (valency of carbon is 4).
- Carbon has the ability to form bonds with its own atoms forming a chain.
- Many compounds of carbon show isomerism.
Question 15.
Explain the tetravalency of carbon atom.
Answer:
The atomic number of carbon is 6. Its electronic configuration is 2, 4. This means an atom of carbon will have to either lose four electrons or gain four electrons to attain the most stable structure. However, both these are difficult.
Therefore, carbon atom overcomes this problem by sharing its four valence electrons with other atoms of carbon or atoms of other elements and attains stable structure. Since four valence electrons are shared, carbon is tetravalent.
Question 16.
Carbon atom does not form C4- anion and C4+ cation. Why?
Answer:
Carbon atom has four electrons in its valence shell. It has to either lose four electrons to form a cation (C4+) or gain four electrons to form an anion (C4-). Both are very difficult as no other atom can spare four electrons nor can an atom of carbon lose four electrons.
Even if a carbon atom gains four electrons, it is difficult for its nucleus to hold on to ten electrons. This is why carbon cannot form either a cation or an anion.
Question 17.
What kind of bonding exists between a carbon atom and atoms of hydrogen, oxygen and sulphur?
OR
Will a carbon atom interact with other atoms to form ionic bonds or covalent bonds?
Answer:
There exists covalent bonding between carbon and other elements such as hydrogen, oxygen and sulphur.
Question 18.
Why are the compounds of carbon generally stable?
Answer:
The strong covalent bonds between carbon atoms make the compounds of carbon highly stable.
Question 19.
What is a covalent bond? What are covalent compounds?
Answer:
A chemical bond formed by the sharing of an electron pair between two atoms is called covalent bond. Compounds formed by covalent bonding are known as covalent compounds.
![]()
Question 20.
Explain the formation of covalent bonding in a hydrogen molecule.
Answer:
The atomic number of hydrogen is 1. It has just one electron in its K shell. It requires one more electron to fill the K shell. Two hydrogen atoms share their electrons to form a molecule of hydrogen, H2. This allows each hydrogen atom to have two electrons in the K shell. Thus, each of the hydrogen atoms attains the stable structure similar to that of helium.

The shared pair of electrons is said to constitute a single bond between the two hydrogen atoms. A single bond is also represented by a line between the two atoms as
H – H
Question 21.
Explain the covalent bond formation between two chlorine atoms in a molecule of chlorine.
Answer:
The atomic number of chlorine is 17. It has seven electrons in its M shell (valence shell). It requires one more electron to get a stable structure. Two chlorine atoms share a pair of electrons to form a molecule of chlorine, Cl2. This allows each chlorine atom to have eight electrons in the M shell.
Thus, each of the chlorine atoms attains the stable structure similar to that of the nearest noble gas. Since the chemical union of two chlorine atoms is achieved by sharing an electron pair, the bonding is covalent in nature.
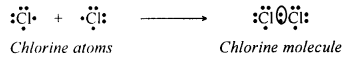
Since the two chlorine atoms share a pair of electrons between them, a single bond is formed between them. This is also represented as follows:
Cl + Cl — Cl–CI → Cl2.
Question 22.
Explain the covalent bond formation between two oxygen atoms in a molecule of oxygen.
Answer:
The atomic number of oxygen is 8. It has six electrons in its L shell (valence shell). It requires two more electrons to get a stable structure. Two oxygen atoms will come together and share two pairs of electrons and form a molecule of oxygen, O2.
This allows each oxygen atom to have eight electrons in the L shell. Thus, each of the oxygen atoms attains the stable structure similar to that of the nearest noble gas. Since the chemical union of two oxygen atoms is achieved by sharing electron pairs, the bonding is covalent in nature.
Since there are two shared pairs of electrons in an oxygen molecule, there exists a double bond between the constituent oxygen atoms.
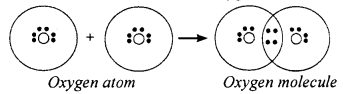
Since the two oxygen atoms share two electrons each, there are two pairs of shared electrons. We say there is a double bond between oxygen atoms in its molecule. This can be written as follows
O + O → O = O → O2
Question 23.
Draw the electron dot structure for the formation of a molecule of ammonia (NH3) and a molecule of methane (CH4).
Answer:
Electron dot structure for the formation of ammonia (NH3)

Question 24.
Explain the electron dot structure of methane Molecule.
Answer:
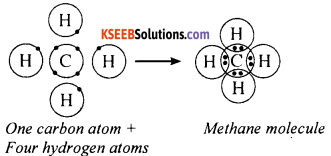
The molecular formula of methane is CH4. The carbon atom has four valence electrons and it needs four more to attain the stable octet. A carbon atom shares its valence electrons with four hydrogen atoms. In the process, both carbon and the four hydrogen atoms attain stable structure.
Question 25.
How many structural isomers can you draw for pentane? What are they?
Answer:
Three different structural isomers can be drawn for pentane. They are as follows
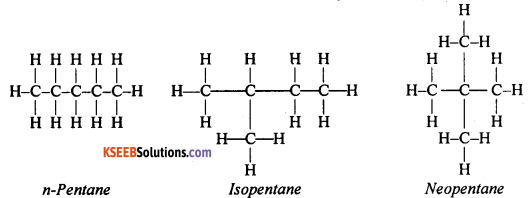
Question 26.
Which is the major constituent of biogas and compressed natural gas (CNG)?
Answer:
The chief constituent of biogas and CNG is methane gas.
Question 27.
Write the electron dot structure for water molecule.
Answer:
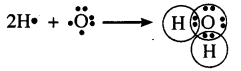
Question 28.
Write the electron dot structure of carbon dioxide (CO2) molecule.
OR
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer:
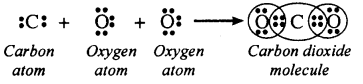
![]()
Question 29.
What would be the electron dot structure of a molecule of sulphur, which is made up of eight atoms of sulphur? (Hint – The eight atoms of sulphur are joined together in the form of a ring.)
Answer:
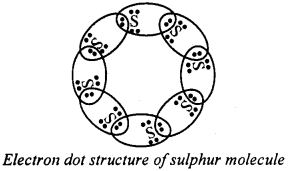
Question 30.
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
Carbon has four valence electrons. In CH3Cl, the carbon atom shares 1 electron each with 3 hydrogen atoms and 1 electron with a chlorine atom. The bond between carbon and chlorine atoms is covalent because it is formed by the sharing of electrons. Similarly, the bond between the carbon atom and each of the hydrogen atoms is also a covalent bond.
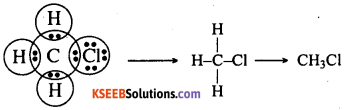
Question 31.
What is allotropy? Name an element that exhibits allotropy.
Answer:
The property of an element to exist in different physical forms with similar chemical properties but different physical properties is called allotropy.
Carbon exhibits allotropy.
Question 32.
Name the three crystalline forms of carbon.
Answer:
The three crystalline forms of carbon are diamond, graphite and fullerene.
Question 33.
Why do we say that diamond and graphite are allotropic forms of carbon?
Answer:
Both diamond and graphite are made up of carbon atoms only. They have similar chemical properties. However, their physical properties are different. Hence, diamond and graphite are called allotropic forms of carbon.
![]()
Question 34.
Name the allotrope of carbon that contains both single and double bonds.
Answer:
Graphite.
Question 35.
What is fullerene?
Answer:
Fullerene is an allotropic crystalline form of carbon. A molecule of fullerene contains 60 atoms of carbon.
Question 36.
What differences do you find in the physical properties of diamond and graphite? What causes these differences?
Answer:
Both diamond and graphite are different physical forms of carbon. Diamond is the hardest substance known while graphite is smooth and slippery. Diamond has much higher density compared to graphite.
Graphite is also a very good conductor of electricity but diamond is an insulator. The difference also lies in the way in which the carbon atoms are bonded to one another. In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. Thus, diamond has a tetrahedral structure while carbon atoms in graphite are arranged in hexagonal layers.
Question 37.
Why do covalent compounds usually have lower melting and boiling points?
Answer:
Covalently bonded molecules of covalent compounds are seen to have strong bonds within the molecule. However, the intermolecular forces within them are small. This explains the low melting and boiling points of such compounds.
Question 38.
Illustrate with suitable examples that compounds of carbon have low melting and boiling points.
Answer:
Carbon is a non-metal. The compounds of non-metals usually have low melting and boiling points. Compounds of carbon also have low melting and boiling points. To illustrate this, let us take the melting and boiling points of four compounds of carbon namely acetic acid, chloroform, ethanol and methane.
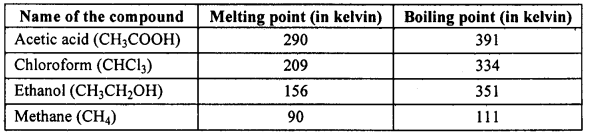
The table above shows that compounds of carbon usually have low melting and boiling points.
Question 39.
List any four properties of covalent compounds.
Answer:
- Covalent compounds are formed by sharing of pairs of electrons between atoms.
- Covalent compounds have low melting and boiling points.
- Covalent compounds do not ionize in solution.
- They are poor conductors of electricity, as they do not contain ions.
Question 40.
Covalent compounds are poor conductors of electricity. Give reason.
Answer:
In covalent compounds molecules are present instead of ions. Due to the absence of ions, these are generally bad conductors of electricity.
Question 41.
What are the two properties of carbon which lead to the huse number of carbon compounds we see around us?
Answer:
The two main properties that enable carbon to have huge number of carbon compounds are:
- Its tetravalency (valency of carbon is 4), and
- Catenation or its ability to form bonds with its own atoms forming a chain.
Question 42.
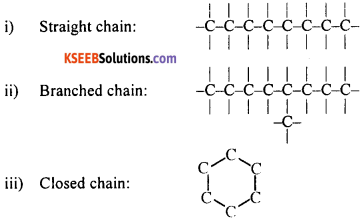
What is catenation? Mention the different ways in which catenation can occur.
OR
Describe catenation by giving suitable examples.
Answer:
Carbon has the ability to form bonds with its own atoms forming a chain. This phenomenon is known as catenation. Catenation can occur in three different ways
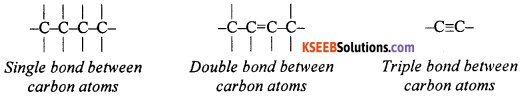
Question 43.
How many bonds are possible between two carbon atoms in a compound? Show by suitable diagrams.
Answer:
There can be one, two or three bonds between carbon atoms in a compound.
Question 44.
What do the bonds between the atoms of carbon represent in an organic compound?
Answer:
The bonds between carbon atoms in an organic compound represent the number of pairs of electrons shared between those two atoms. A single bond indicates that one pair of electrons is shared. A double bond indicates that two pairs of electrons are shared. Likewise, a triple bond indicates that three pairs of electrons are shared between the two carbon atoms.
![]()
Question 45.
What are hydrocarbons? What are saturated and unsaturated hydrocarbons?
Answer:
Compounds of carbon with hydrogen are called hydrocarbons. Hydrocarbons in which there is only a single bond between carbon atoms are called saturated hydrocarbons. Hydrocarbons in which there is either a double bond or a triple bond between the carbon atoms are called unsaturated hydrocarbons.
Question 46.
Mention four differences between saturated and unsaturated hydrocarbons.
Answer:
| Saturated hydrocarbons | Unsaturated hydrocarbons |
| 1. Only single bond is present between carbon- carbon. | Double or triple bond is also present between carbon-carbon. |
| 2. Substitution reaction occurs. | Addition reaction occurs. |
| 3. It burns with blue flame. | It burns with sooty flame. |
| 4. Less reactive. | Highly reactive. |
Question 47.
With how many monovalent atoms can a carbon atom combine? Why?
Answer:
A carbon atom can combine with four monovalent atoms. This is because the valency of carbon is 4.
Question 48.
Why are compounds of carbon highly stable?
Answer:
In compounds of carbon the bond between carbon atoms and the bonds between carbon and other atoms are very strong. This is why compounds of carbon are highly stable. One reason for the formation of strong bonds by carbon is its small size. This enables the nucleus to hold on to the shared pairs of electrons strongly.
Question 49.
- What is organic chemistry?
- What are organic compounds?
Answer:
- The branch of chemistry that deals with carbon compounds excluding simple salts such as carbonates, oxides, and carbides is called organic chemistry.
- The compounds of carbon that contain at least one C-H bond are called organic compounds.
Question 50.
Who disproved the theory that organic compounds can be obtained only from living sources? How did he do it?
Answer:
Friedrich Wohler, a German chemist, disproved the theory that organic compounds can be obtained only from living sources. He did this in 1828 by preparing urea (an organic compound) from ammonium cyanate (non-living source).
Question 51.
Give the name, formula and structure of first six compounds of carbon and hydrogen which have only single bonds between their carbon atoms.
Answer:
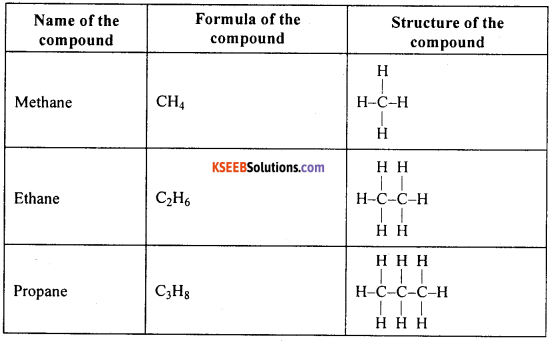
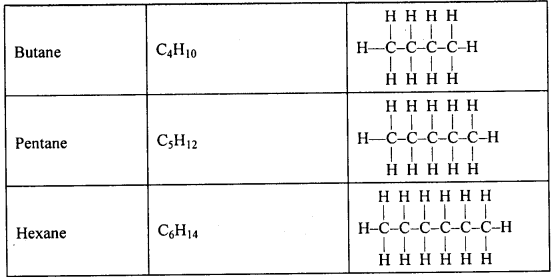
Question 52.
Write the electron dot structure of ethane.
Answer:
![]()
Question 53.
Write the name, molecular formula and structural formula of the first three compounds of carbon and hydrogen having a double bond between their carbon atoms.
Answer:
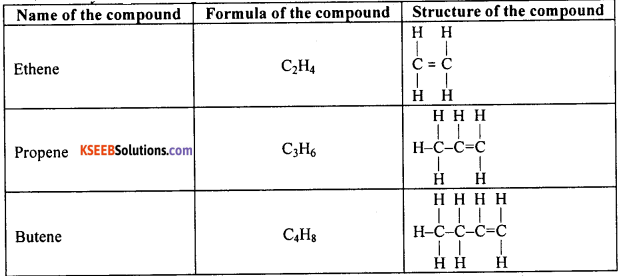
Question 54.
Write the name, molecular formula and structural formula of the first three compounds of carbon and hydrogen having a triple bond between their carbon atoms.
Answer:
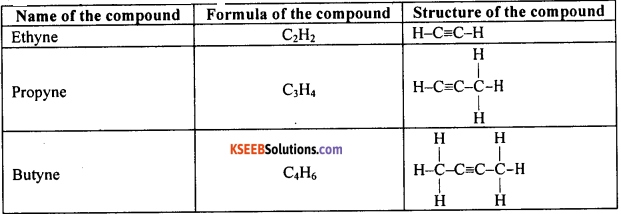
Question 55.
What is isomerism? What are isomers? Write the structures of isomers of butane.
Answer:
The existence of two or more different organic compounds with the same molecular formula but different physical and chemical properties is called isomerism.
The organic compounds having the same molecular formulae but different structural formulae are called isomers. The molecular formula of butane is C4H10. It has two isomers namely Normal butane and Isobutane. The structural formulae of these two compounds are as follows
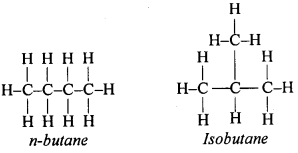
Question 56.
What are structural isomers? Name the first member of alkanes that shows structural isomerism.
Answer:
Organic compounds having the same molecular formulae but different structural formulae are called structural isomers. Butane is the first alkane that shows structural isomerism.
Question 57.
What are cyclic compounds? Give two examples with their molecular formula and structural formula.
Answer:
A cyclic compound is a compound in which a series of atoms connect to form a ring. eg: Cyclopropane, Cyclobutane, Cyclopentane, Cyclohexane etc.
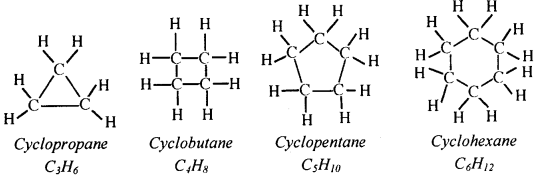
Question 58.
What will be the formula and electron dot structure of cyclopentane?
Answer:
The molecular formula of cyclopentane is C5H10. The electron dot structure of this compound is given below
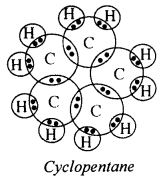
Question 59.
Write the electron dot structure of cyclohexane.
Answer:
The molecular formula of cyclohexane is C6H12. Its electron dot structure and structural formulae are shown below
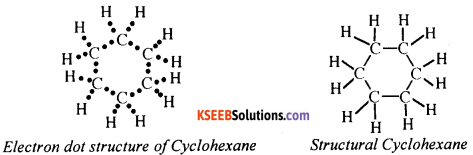
Question 60.
Draw the electron dot structures for
- ethanoic acid,
- H2S2
- propanone, and
- F2
Answer:
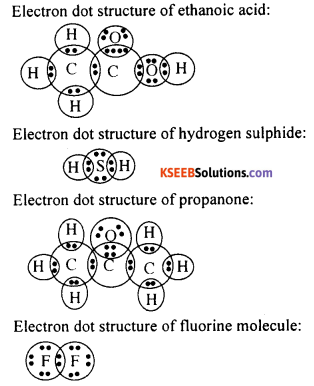
Question 61.
Give an example of a cyclic compound with double bonds between its carbon atoms.
Answer:
Benzene is an example of a cyclic compound with double bonds between some of its carbon atoms. Its molecular formula is The ring structure of benzene is
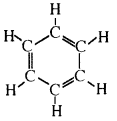
Question 62.
What are hydrocarbons?
Answer:
Compounds containing only carbon and hydrogen are called hydrocarbons.
![]()
Question 63.
How are hydrocarbons broadly classified?
Answer:
Hydrocarbons are broadly classified into two groups as
- saturated hydrocarbons and
- unsaturated hydrocarbons.
Question 64.
What are alkanes? Give two examples.
Answer:
Saturated open chain hydrocarbons, which have only a single bond between their carbon atoms, are called alkanes.
eg: Methane (CH4), Ethane (C2H6), Propane (C3H8) etc.
Question 65.
What are alkenes? Give two examples.
Answer:
Unsaturated open chain hydrocarbons, which have at least one double bond between their carbon atoms, are called alkenes.
eg: Ethene (C2H4), Propene (C3H6), Butene (C4H8) etc.
Question 66.
What are alkynes ? Give two examples.
Answer:
Highly unsaturated open chain hydrocarbons, which have at least one triple bond between their carbon atoms are called alkynes.
eg: Ethyne (C2H2), Propyne (C3H4), Butyne (C4H6) etc.
Question 67.
Distinguish between saturated hydrocarbons and unsaturated hydrocarbons.
Answer:
Saturated hydrocarbons:
- These compounds have only a single bond between their carbon atoms.
- These compounds give substitution reactions.
Unsaturated hydrocarbons:
- These compounds have at least one double or a triple bond between their carbon atoms.
- These compounds give addition reactions.
Question 68.
Write the structure of the simplest hydrocarbon.
Answer:
The simplest hydrocarbon is methane (CH4). Its structure is
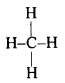
Question 69.
Write the molecular formula and structural formula of an alkene having five carbon atoms.
Answer:
The alkene with 5 atoms of carbon is called pentene. Its molecular formula is C5H10. The structural formula of pentene is given below
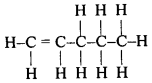
Question 70.
What is a heteroatom? Explain.
Answer:
Carbon combines with not only hydrogen but also with other elements such as halogens, oxygen, nitrogen and sulphur. In a hydrocarbon chain, one or more hydrogen atoms can be replaced by atoms of these elements. In such compounds, the element replacing hydrogen is referred to as a heteroatom.
In organic chemistry, heteroatoms are atoms of other elements that replace one or more hydrogen or carbon atoms in an organic compound, such that the overall valency remains satisfied.
![]()
Question 71.
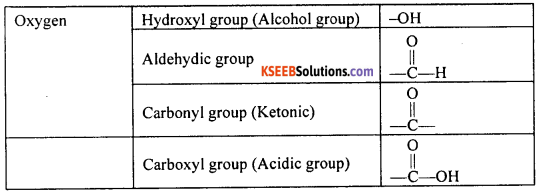
What are functional groups? List some of the common functional groups found in organic compounds. Give their group formula.
Answer:
The group of heteroatoms in an organic compound that determines the chemical behaviour of the compound is called a functional group. Some of the common functional groups found in compounds of carbon are given in the table below

Question 72.
What is a homologous series? Explain with an example.
Answer:
A group of organic compounds, which can be represented by the same general formula, have the same functional group and similar chemical properties constitute a homologous series. The successive members of the series differ by a CH2 group and 14 mass units.
The chemical properties of methanol (CH3OH), Ethanol (C2H5OH), Propanol (C3H7OH) and Butanol (C4H9OH) are all very similar. All these compounds can be represented by the same general formula CnH2n+1OH (R-OH) where n represents the number of carbon atoms in their molecule.
All these compounds have the same functional group namely -OH. Successive members of the series differ by CH2 group. Therefore, these compounds belong to the same family and constitute a homologous series. The name of the homologous series is alcohols.
Question 73.
List the characteristics of a homologous series.
Answer:
The following are the characteristics of the members of a homologous series
- All the members of a homologous series can be represented by the same general formula.
- Successive members differ from each other by -CH2 group.
- Physical properties show a regular gradation with increasing number of carbon atoms.
- Members have similar chemical properties because they have same functional group.
- Members of the homologous series can be prepared using similar method.
Question 74.
Name six different homologous groups of organic compounds.
Answer:
Homologous groups of organic compounds include alkanes, alkenes, alkynes, aromatic hydrocarbons, cycloalkanes, alcohols, aldehydes, ketones, etc.
![]()
Question 75.
Explain the meaning of homologous series by taking alkynes as an example.
Answer:
Homologous series refers to a group of related compounds that have similar chemical properties and carry the same functional group. Two successive members of a homologous series differ by -CH2 group. Let us consider the homologous series of alkynes.
The members of this series in order are ethyne (C2H2), propyne (C3H4), butyne (C4H6), pentyne (C5H8), hexyne (C6H10) etc. We observe that two successive members of this series differ by -CH2 group. The physical properties show a regular gradation as we move towards the higher end of the series.
These compounds have similar chemical properties and can be prepared by similar methods. These compounds have the same general formula CnH2n-2. Thus, alkynes constitute a homologous series.
Question 76.
Write the molecular formula of the following and show that they form homologous series
- Methanol
- Ethanol
- Propanol.
Answer:
The molecular formula of methanol, ethanol and propanol are given below
| Name of the compound | Molecular formula |
| Methanol | CH3OH |
| Ethanol | C2H5OH |
| Propanol | C3H7OH |
All the three compounds listed here have the same functional group namely -OH (hydroxyl group). The successive members of the group differ by CH2. They can be represented by the same general formula CnH2n+1-OH. They have similar chemical properties. Hence methanol, ethanol and propanol are members of the same homologous series called ‘alcohols’.
Question 77.
The molecular formula of a homologue is C5H12. Write the molecular formulae of the next four members of the series. What is the general formula of these compounds? What is the homologous series called?
Answer:
The molecular formulae of the next four members of the series are C6H14, C7H16, C8H18, and C9H20. The general formula of these compounds is CnH2n+2. The name of the homologous series is ‘alkanes’.
Question 78.
Calculate the difference in the formulae and molecular masses for
- CH3OH and C2H3OH
- C2H5OH and C3H7OH
- C3H7OH and C4H9OH.
Is there any similarity in these three? Arrange these alcohols in the order of increasing carbon atoms to get a family. Can we call this family a homologous series?
Answer:
1. The difference in the formulae for CH3OH and C2H5OH is CH2. The difference in the masses of these compounds is 14.
2. The difference in the formulae for C2H5OH and C3H7OH is CH2. The difference in the masses of these compounds is 14.
3. The difference in the formulae for C3H7OH and C4H9OH is CH2. The difference in the masses of these compounds is 14.
The arrangement of the given alcohols in the increasing order of the number of carbon atoms is CH3OH, C2H5OH, C3H7OH, and C4H9OH. Yes, we can call this family a homologous series.
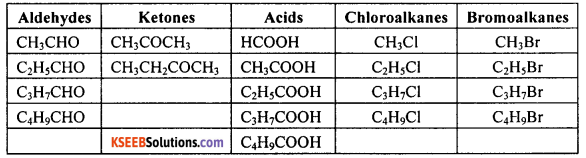
Question 79.
Generate the homologous series for compounds containing up to four carbon atoms for the functional groups such as aldehydes, ketones, acids, chloroalkanes and bromoalkanes.
Answer:
![]()
Question 80.
Describe the method of naming organic compounds.
Answer:
Carbon compounds are named by following the method given below:
1. First, the number of carbon atoms in the compound is identified. A compound with one carbon atom would have the name ‘methane’, a compound with two carbon atoms would have the name ‘ethane’, a compound with three carbon atoms would have the name propane and so on.
2. In case a functional group is present, it is indicated in the name of the compound either by prefixing or suffixing it.
3. If the name of the functional group is given in suffix, the name of the carbon chain is modified by deleting the final ‘e’ and adding the appropriate suffix. For example, a three-carbon chain with a ketone group would be named in the following manner: Propane – ‘e’ = propan + ‘one’ = propanone.
4. If the carbon chain is unsaturated and has a double bond, then the final ‘ane’ in the name of the carbon chain is substituted by ‘ene’. In case of a triple bond, ‘ane’ is replaced by ‘yne’. For example, a three-carbon chain with a double bond would be called propene and if it has a triple bond, it would be called propyne.
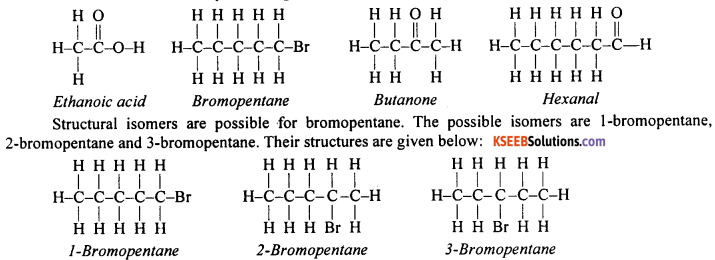
Question 81.
Draw the structures for the following compounds
- Ethanoic acid
- Bromopentane,
- Butanone
- HexanaL
Are structural isomers possible for bromopentane?
Answer:
The structure of the compounds is given below
Question 82.
How would you name the following compounds?
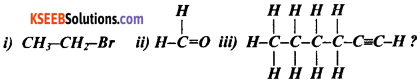
Answer:
(i) This compound is obtained by replacing one hydrogen atom of ethane by an atom of bromine. The functional group in the compound is bromine. The prefix therefore is ‘bromo’ before ethane. Thus the name of the compound is bromoethane.
(ii) This compound contains -CHO group i.e., the ‘aldehyde’ group. The suffix for aldehyde group is ‘al’. The molecule contains only one carbon atom. Therefore the name should contain ‘methane’. Thus, the name of the compound is methanal.
(iii) This compound is a hydrocarbon with 6 carbon atoms. Its name should contain ‘hex’. The compound also has a triple bond. Therefore, the name of the compound should end with ‘yne’. Thus, the name of the compound is hexyne.
Question 83.
What are the suffixes to be added while naming the organic compounds with the following functional groups: alcohol, aldehyde, ketone, carboxylic acid, alkane, alkene, alkyne?
Answer:
The suffixes to be added while naming the organic compounds are given in the table below
| Functional group | Suffix to be added |
| Alcohol (-OH) | -ol |
| Aldehyde (-CHO) | -al |
| Ketone (C=0) | -one |
| Carboxylic acid (-COOH) | -oic acid |
| Alkane (single bond) | – ane |
| Alkene (double bond) | – ene |
| Alkyne (triple bond) | – vne |
Question 84.
Name the functional group in the following compounds and write their molecular formula
- Ethanol
- Ethanoic acid.
Answer:
1. Ethanol: Functional group present in ethanol is alcohol (-OH).
Molecular formula is C2H5OH.
2. Ethanoic acid: Functional group present in ethanoic acid is carboxylic acid (-COOH). Molecular formula is CH3COOH.
![]()
Question 85.
Write the structural formula of the following compounds: propanol, propanal, propanone, propanoic acid, propane, propene and propyne.
Answer:
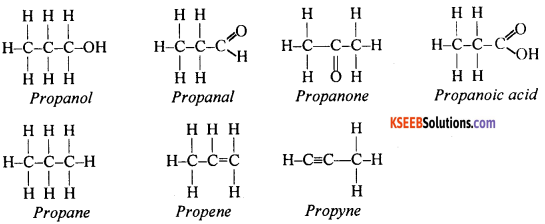
Question 86.
How do carbon compounds react with oxygen? Give examples with equations.
Answer:
Compounds of carbon, just like carbon, burn in air (oxygen) and give carbon dioxide along with heat and light.

Question 87.
Why are carbon and its compounds used as fuels for most applications?
Answer:
Most of the carbon compounds give a lot of heat and light when burnt in air. Saturated hydrocarbons burn with a clean flame and no smoke is produced. The carbon compounds, used as fuel, have high calorific values. Therefore, carbon and its compounds are used as fuels for most applications.
Question 88.
Explain the effect of ethanol and methanol on our body.
Answer:
When large quantities of ethanol are consumed, it tends to slow metabolic processes and to depress the central nervous system. This results in lack of coordination, mental confusion, drowsiness, lowering of the normal inhibitions, and finally stupour.
Unlike ethanol, intake of methanol in very small quantities can cause death. Methanol is oxidised to methanal in the liver. Methanol reacts rapidly with the components of cells. It coagulates the protoplasm. Methanol also affects the optic nerve, causing blindness.
Question 89.
It is said that one should not mix drinking with driving. Why?
Answer:
Alcohol slows down the cognitive response of the body. Driving is a task that requires a high level of alertness and very good hand-eye coordination. All of this would be absent if a person is under the influence of alcohol. It may result in a fatal accident, which may put to risk the life of the driver, fellow passengers and other road users. Hence, it is said that one should not mix drinking with driving.
Question 90.
What is denatured alcohol?
Answer:
The alcohol (ethanol) used for industrial purposes is made unfit for drinking by adding certain poisonous substances such as methanol and some dyes. This is called methylated spirit or denatured alcohol.
Question 91.
What would happen if somebody consumed denatured alcohol?
Answer:
When large quantities of ethanol are consumed, it tends to slow metabolic processes and to depress the central nervous system. This results in lack of coordination, mental confusion, drowsiness, lowering of the normal inhibitions, and finally stupour.
![]()
Question 92.
What is oxidation? What are oxidising agents? Give two examples.
Answer:
The process in which a chemical substance changes because of the addition of oxygen is called oxidation. Chemical substances that are capable of adding oxygen to other chemical substances are known as oxidising agents. eg: Potassium permanganate, nitric acid, hydrogen peroxide etc.
Question 93.
Show that saturated hydrocarbons burn with a non-luminous flame while unsaturated hydrocarbons burn with a luminous sooty flame.
Answer:
Take some hydrocarbons such as naphthalene, camphor, alcohol etc. Heat each of these compounds one by one on a spatula and burn them. Place a metal plate above the flame. Saturated hydrocarbons like alcohol burn with a blue flame and produce no smoke or soot.
Unsaturated hydrocarbons such as naphthalene and camphor burn with a luminous yellow flame and also produce soot which gets collected on the metal plate. This shows that saturated hydrocarbons burn with a non-luminous flame while unsaturated hydrocarbons burn with a luminous sooty flame.
Question 94.
Under what condition do saturated hydrocarbons also produce smoke and soot?
Answer:
Saturated hydrocarbons burn and produce smoke and soot when they are made to undergo combustion in limited supply of oxygen. However, they bum with a clear blue flame in excessive supply of oxygen.
Question 95.
How do you show that incomplete combustion of a fuel produces a yellow sooty flame whereas complete combustion produces a blue flame?
Answer:
Light a Bunsen burner. Reduce the air hole at the base of the burner to decrease the supply of air. Observe the colour of the flame. Also place a metal plate over the flame and see whether there is any collection of soot. Now increase the size of the hole to ensure enough supply of air.
Observe the colour of the flame and check whether there is any production of soot. The flame bums with a sooty yellow flame when the supply of air is limited. However, the flame is clear blue and smoke-free when adequate supply of air is ensured.
Question 96.
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer:
Ethyne is an unsaturated hydrocarbon with high carbon content. It bums in air with a sooty flame, undergoes incomplete combustion due to inadequate supply of oxygen. Therefore, heat produced is also not sufficient to attain high temperature required for welding.
When ethyne is burnt in oxygen, the combustion is complete and high temperature is attained due to the production of large amount of heat. This is the temperature ideal for welding. Therefore, a mixture of ethyne and oxygen is burnt for welding work.
2 HC ≡ CH + 5 O2 → 4 CO2 + 2H2O + Heat and light.
Question 97.
Describe an experiment to show the oxidation reaction between alcohol and alkaline potassium permanganate. Represent the reaction with a suitable equation.
Answer:
Take about 3 mL of ethanol in a test tube. Warm the compound gently in a water bath. Add a 5% solution of alkaline potassium permanganate drop by drop to this solution and observe the changes. The colour of potassium permanganate is found to disappear initially. This is because KMNO4 is used to oxidise ethanol to ethanoic acid.
During the process, potassium permanganate is reduced to manganese dioxide. When KMNO4 is added in excess, the colour is retained as there will be some amount of unused potassium permanganate in the solution. This activity shows that potassium permanganate oxidises ethanol to ethanoic acid arid in the process gets reduced to manganese dioxide.
![]()
Question 98.
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer:
The conversion of ethanol to ethanoic acid involves addition of oxygen atoms to ethanol. Therefore, this process is considered an oxidation reaction.
Question 99.
Write the equation of the chemical reaction taking place when ethanol is heated with alkaline potassium permanganate. Name the product.
Answer:
Alkaline potassium permanganate is an oxidizing agent. On heating with ethanol, potassium permanganate oxidizes ethanol to ethanoic acid.
C2H5OH + KMnO4 → CH3COOH + MnO2
Question 100.
Name the catalysts commonly used in hydrogenation of oils to form fats.
OR
Name the catalysts used in the reaction to obtain saturated hydrocarbons from unsaturated hydrocarbons.
Answer:
Nickel and Palladium.
![]()
Question 101.
What are addition reactions? Explain with an example.
Answer:
A chemical reaction in which one or more atoms get added to a compound resulting in a larger molecule of another compound is called addition reaction. Addition reactions are a characteristic feature of unsaturated compounds.
For example, ethene is an unsaturated hydrocarbon having a double bond between its carbon atoms. This compound reacts with bromine to form another compound called dibromoethane. In this process, two bromine atoms get added to the molecule of ethene. This reaction gives rise to a saturated compound called dibromoethane.
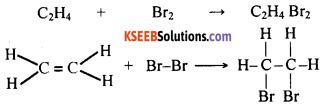
Question 102.
Observe the following reactions
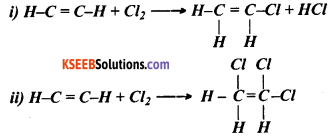
State whether these reactions are addition reactions or substitution reactions. Justify your answer.
Answer:
The chemical reactions given here are not substitution reactions. They are addition reactions. This is because in these reactions chlorine atoms are being added to the reactants and they are not replacing any other atom during the process.
In the given chemical reactions, there is a double bond between the carbon atoms. Therefore, these compounds are unsaturated. Usually, unsaturated hydrocarbons give addition reactions. Both the reactions given here are addition reactions.
Question 103.
Give one industrial application of addition reaction.
Answer:
Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons. Such a reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst.
Vegetable oils generally have long unsaturated carbon chains. These oils are converted into fats by adding hydrogen. This process turns unsaturated carbon chains into saturated chains by adding hydrogen atoms.
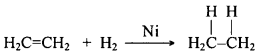
Question 104.
What is hydrogenation? What is its industrial application?
Answer:
Addition of hydrogen to an unsaturated carbon compound is called hydrogenation. In industries, hydrogenation reaction is used for making vegetable ghee from vegetable oils. Vegetable oils such as groundnut oil, cottonseed oil, etc., are unsaturated compounds, which contain double bonds in their molecules.
They are converted into ghee by hydrogenation in the presence of a suitable catalyst such as nickel or platinum. The process of converting a vegetable oil into solid fat is called hydrogenation of oil.
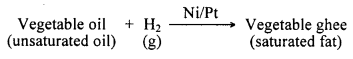
Question 105.
Name the reaction which is commonly used in the conversion of vegetable oils to fats.
Answer:
The reaction used in the conversion of vegetable oils to fats is known as hydrogenation reaction.
Question 106.
Which of the following hydrocarbons undergo addition reactions: C2H6, C3H8, C3H6, C2H2 and CH4?
Answer:
Of the given compounds C2H6, C3H8 and CH4 are saturated hydrocarbons. These compounds have only a single bond between the carbon atoms. Therefore, these compounds do not give addition reactions. Compound such as C3H6 and C2H2 are unsaturated hydrocarbons having a double bond and a triple bond respectively between their carbon atoms. Therefore, these two compounds give addition reactions.
Question 107.
Oils and not fats should be chosen for cooking. Give reason.
Answer:
Fats contain saturated fatty acids that are harmful to health. Oils however contain unsaturated fatty acids and are healthy and hence should be chosen for cooking.
Question 108.
Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer:
Cooking oil is an unsaturated fatty acid. Cooking oil undergoes addition reactions such as hydrogenation. Butter is a saturated fatty acid. It does not undergo addition reactions such as hydrogenation.
Question 109.
What is substitution reaction? Explain with an example and chemical equation.
Answer:
A chemical reaction in which an atom or a group of atoms in a compound is replaced by another atom or group of atoms is called a substitution reaction. For example, when a mixture of methane and chlorine is kept in sunlight, chlorine atoms progressively replace the hydrogen atoms of methane.
The products obtained during the reaction depend on the number of hydrogen atoms substituted by chlorine atoms. We get chloromethane, dichloromethane, trichloromethane and tetrachloromethane respectively. The equations of the reactions are as follows:
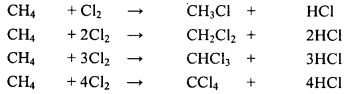
Question 110.
Alkanes undergo only substitution reactions but alkenes and alkynes undergo both substitution and addition reactions. Why?
Answer:
Alkanes are saturated hydrocarbons as they have only single bond between their carbon atoms. Therefore, they give only substitution reactions. Alkenes and alkynes are unsaturated hydrocarbons. Hence they give both substitution and addition reactions.
Question 111.
What is ethanol? What is its molecular formula and structural formula?
Answer:
An organic compound that is colourless, volatile and flammable, which is produced by the natural fermentation of sugars and forms an active component of intoxicating drinks is called ethanol. It is also called ethyl alcohol. The molecular formula of ethanol is CH3CH2OH. Its structural formula is:
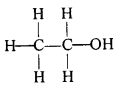
Question 112.
How does ethanol react with sodium metal? Write a balanced equation for the reaction.
OR
Which gas is evolved when ethanol reacts with sodium? Name the gas evolved and also write the balanced chemical equation of the reaction involved.
Answer:
Ethanol reacts with sodium forming sodium ethoxide. Hydrogen gas is evolved during this reaction.
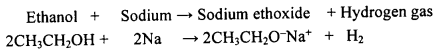
Question 113.
How is ethene produced from ethanol? Give balanced chemical equation for the reaction.
Answer:
When heated at 443 K with excess of concentrated sulphuric acid, ethanol gets dehydrated and forms ethene and water.
![]()
Question 114.
You are given ethanol, concentrated sulphuric acid and a small piece of sodium metal. Using these chemicals, how do you prepare
- Hydrogen gas
- Ethene gas?
Answer:
1.
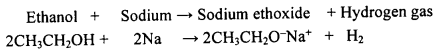
2.
![]()
Question 115.
Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid. What is the role of sulphuric acid in this reaction? Write the balanced chemical equation of this reaction.
Answer:
When ethanol is heated with excess of concentrated sulphuric acid at 443 K, it gets dehydrated to form ethene.

In this reaction, concentrated sulphuric acid acts as a dehydrating agent that removes water molecule from the ethanol molecule.
Question 116.
List the uses of ethanol.
Answer:
- Ethanol is an active ingredient in all intoxicating drinks.
- It is used as a fuel.
- It is used as a solvent.
- It is also used in medicines such as tincture iodine, cough syrups, and many tonics.
![]()
Question 117.
What is vinegar? Mention one of its uses.
Answer:
5-8% solution of acetic acid in water is called vinegar. It is used widely as a preservative in pickles.
Question 118.
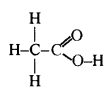
What is ethanoic acid commonly called? What is its molecular formula? Write its structural formula.
Answer:
Ethanoic acid is commonly called acetic acid. Its molecular formula is CH3COOH. Its structural formula is
Question 119.
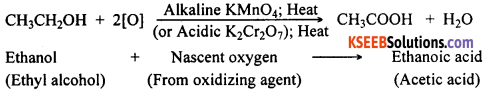
How can ethanol be converted into ethanoic acid?
Answer:
When ethanol is heated with alkaline potassium permanganate or acidic potassium dichromate, ethanol gets oxidised to ethanoic acid. The reaction can be written as below
Question 120.
What is glacial acetic acid?
Answer:
The melting point of pure ethanoic acid is 290 K and hence it often freezes during winter in cold climates. This is called glacial acetic acid.
Question 121.
How do you practically compare the strength of acetic acid and dilute hydrochloric acid?
Answer:
Compare the pH of dilute acetic acid and dilute hydrochloric acid using both litmus paper and universal indicator. Both dilute hydrochloric acid and acetic acid turn blue litmus red. This test is not suitable to test the relative strength of the two acids. When tested with universal indicator, the indicator turns yellowish pink indicating that the pH value of acetic acid is around 4.
In hydrochloric acid, the universal indicator turns light pink indicating a pH value of 2. This shows that hydrochloric acid is stronger than acetic acid. HCl gets completely ionised in solution whereas acetic acid gets partially ionized. This again shows that acetic acid is a weaker acid while hydrochloric acid is a stronger acid.
Question 122.
What is an ester? Give an example.
Answer:
A compound produced by the reaction between an acid and an alcohol with the elimination of a molecule of water is known as ester. eg: Ethanoic acid reacts with ethanol forming ethyl ethanoate along with water. Here ethyl ethanoate is an ester.
Question 123.
Show the formation of an ester from ethanol.
Answer:
Take 1 mL ethanol (absolute alcohol) and 1 mL glacial acetic acid along with a few drops of concentrated sulphuric acid in a test tube. Warm the contents in a water-bath for at least five minutes. Pour into a beaker containing 20-50 mL of water and smell the resulting mixture.
Absolute ethanol reacts with glacial acetic acid in the presence of a few drops of sulphuric acid to form a sweet-smelling compound called ethyl ethanoate. This is an ester.
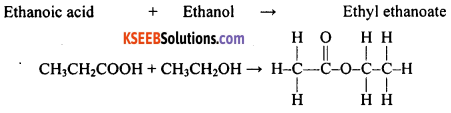
Question 124.
How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer:
We can distinguish between an alcohol and a carboxylic acid on the basis of their reaction with carbonates and hydrogen carbonates. Carboxylic acids react with carbonate and hydrogen carbonate producing CO2 gas that turns lime water milky. Alcohols, on the other hand, do not react with carbonates and hydrogen carbonates.
There is another way to distinguish between alcohol and carboxylic acid. Alcohol is highly inflammable while carboxylic acid is non-inflammable. This property can be utilised to differentiate the given samples. The substance that catches fire is an alcohol and the substance that does not catch fire is a carboxylic acid.
Question 125.
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer:
Ethanol is a pleasant-smelling liquid while ethanoic acid has the smell of vinegar. Ethanoic acid solidifies easily in cold conditions whereas ethanol does not. Ethanoic acid turns blue litmus red while ethanol does not change the colour of litmus.
Ethanol does not react with sodium hydrogen carbonate where as ethanoic acid reacts briskly with sodium hydrogen carbonate giving out carbon dioxide gas.
Question 126.
List the uses of ethanoic acid.
Answer:
The following are some of the uses of ethanoic acid
- Vinegar is used as a preservative in pickles. It is also used as a food additive in many dishes.
- Ethanoic acid is used for the production of polymers.
- Ethanoic acid is used for the production of esters. Esters are sweet-smelling substances, which are used as food additives.
Question 127.
What is esterification?
Answer:
When an alcohol reacts with an organic acid in the presence of acid catalyst, a sweet-smelling liquid called ester is obtained. This reaction is called esterification.
Question 128.
Give two uses of esters.
Answer:
Esters are used in making perfumes and as flavouring agents.
![]()
Question 129.
Explain the reaction of an ester with sodium hydroxide. Write suitable equation for the reaction.
Answer:
On treating with sodium hydroxide, an ester is converted into alcohol and sodium salt of carboxylic acid.
![]()
Question 130.
What is saponification? Give an example. Where is this reaction used?
Answer:
The hydrolysis of a fat by an alkali, which results in the formation of soap and glycerol, is called saponification. Ethyl ethanoate is an ester. It reacts with sodium hydroxide and forms ethanol and sodium salt of the carboxylic acid. This is an example of saponification. Saponification reactions are used in the manufacture of soap.
Question 131.
Explain the reaction between ethanoic acid and a base such as sodium hydroxide.
Answer:
Ethanoic acid reacts with a base such as sodium hydroxide to give a salt called sodium ethanoate and water.
NaOH + CH3COOH → CH3COONa + H2O
Question 132.
How does carboxylic acid react with sodium carbonate and sodium bicarbonate? Explain.
Answer:
Carboxylic acid is also called ethanoic acid. Ethanoic acid reacts with sodium carbonate and gives a salt, carbon dioxide and water. It reacts similarly with sodium bicarbonate. The reactions can be written as follows
Na2CO3 + 2 CH3COOH → 2 CH3COONa + CO + H2O
NaHCO + CH3COOH → NaCH3COO + H2O + CO2
Question 133.
How do you establish that the reaction between carboxylic acids and sodium carbonate (or sodium hydrogen carbonate) liberates carbon dioxide gas?
Answer:
Ethanoic acid is a carboxylic acid. Take a spatula full of sodium carbonate in a test tube and add 2 mL of dilute ethanoic acid. Immediately we observe the evolution of a gas. This gas is known to turn lime water milky. This shows that carbon dioxide is evolved during the reaction. Similar changes occur when the activity is repeated with sodium hydrogen carbonate.
Na2CO3 + 2 CH3COOH → 2 CH3COONa + CO + H2O
NaHCO + CH3COOH → NaCH3COO + H2O + CO2
Question 134.
What are soaps? How are they prepared?
Answer:
Sodium salts or potassium salts of long chain carboxylic acids are called Soaps. Soaps are prepared by the hydrolysing of oils and fats with a suitable alkali.
Question 135.
Show experimentally that oil dissolves in soap solution.
Answer:
Take about 10 mL of water in each of the two test tubes. Add a drop of cooking oil to both the test tubes and label them as A and B. To test tube B, add a few drops of soap solution. Now shake both the test tubes vigorously for the same period of time. Leave the test tubes undisturbed for some time.
Observe the two test tubes. It is observed that in test tube A, water and cooking oil form two separate layers. In test tube B, however, we observe just one layer. This shows that oil dissolves in soap and thus soap helps in cleaning.
Question 136.
Soap can mix with both water and with oil. Why?
Answer:
Soap molecule has two different ends. One of the ends is the ionic end that binds with water. The other end is the non-polar hydrocarbon end which binds with grease and oil. The ionic end dissolves in water while the hydrocarbon end dissolves in oil. Thus soap can mix with both water and oil.
Question 137.
How does soap help to dissolve dirt in water?
OR
Explain the cleansing action of soap.
Answer:
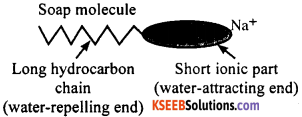
The molecules of soap are sodium salts or potassium salts of long-chain carboxylic acids. A molecule of soap has two ends namely the ionic end and the hydrocarbon end. The ionic end of soap dissolves in water while the hydrocarbon end dissolves in oil.
The soap molecules thus form structures called micelles where one end of the molecules is towards the oil droplets while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in dissolving the dirt in water and wash our clothes clean.
![]()
Question 138.
What are micelles? Show it by a diagram.
Answer:
When greasy dirt or oil is mixed with soapy water, a structure is formed in which the ionic end of soap molecules sticks to water and point outwards while the molecule end sticks to the oil in the centre where it can’t come in contact with water. This structure is called soap micelles.
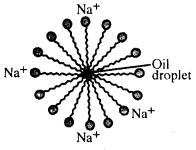
Question 139.
Why does soap solution appear cloudy when oil or dirt is mixed in it?
Answer:
When greasy dirt or oil is mixed with soapy water, a structure called micelles is formed. The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.
Question 140.
What is hard water? What is the hardness of water due to?
Answer:
Water sample that does not give free lather with soap is called hard water. Water that contains dissolved salts such as bicarbonates, chlorides or sulphates is called hard water. Water becomes hard due to dissolved bicarbonates, chlorides and sulphates.
Question 141.
How do you test whether a given sample of water is hard or not?
Answer:
Take about 10 mL of the given sample of water in a test tube. Add a couple of drops of soap solution. Shake the test tube vigorously. If the solution forms lather freely, the given sample of water is soft. If it gives a white curdy precipitate, the water is hard.
Question 142.
Why doesn’t soap clean clothes well in hard water?
Answer:
Soap molecules have an ionic end and a non-ionic hydrocarbon end. The ionic end of the soap molecule combines with salts present in hard water and forms scum. This is why soap does not clean well with hard water.
Question 143.
What are detergents?
Answer:
Detergents are synthetic cleansing agents and are generally ammonium or sulphonate salts of long chain carboxylic acids. They clean well even in hard water.
Question 144.
Would you be able to check if water is hard by using a detergent?
Answer:
Both hard water and soft water give free lather with detergents. Soaps give free lather with soft water but not with hard water. Therefore, detergents cannot be used to check the hardness of water.
![]()
Question 145.
People use a variety of methods to wash clothes. Usually, after addins soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washine machine. Why is aeitation necessary to set clean clothes?
Answer:
When micelles are formed after adding soap, they stick to the surface of the cloth. When the cloth is agitated, micelles get separated from cloth, which can be washed away easily. Hence, agitation is necessary to wash clothes.
Question 146.
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer:
Soap is sodium or potassium salt of long chain fatty acid. The two ends of soap molecules have different properties. The ionic end is hydrophilic. It dissolves in water while the hydrocarbon end, which is hydrophobic, dissolves in oil. The hydrocarbon end is oriented towards oil droplet while the ionic end is oriented towards water.
This results in the formation of a structure called micelles. No, micelles are not formed in other solvents such as ethanol, because ethanol and other compounds act as solvent for hydrophilic as well as hydrophobic end of the soap.
Question 147.
Explain the formation of scum when hard water is treated with soap.
Answer:
Hard water contains hydrogen carbonates, chlorides and sulphates of calcium and magnesium, which react with soap to form scum. For example, calcium chloride reacts with soap to form a whitish precipitate of calcium stearate, which appears as scum.
Sodium stearate + Calcium chloride → Sodium chloride + Calcium stearate (scum)
Question 148.
What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
Soap is alkaline or basic in nature. Therefore, soaps turn red litmus blue. However, blue litmus paper remains blue in soap solution.
Question 149.
Explain the mechanism of the cleanine action of soap.
Answer:
Soaps are sodium or potassium salts of long-chain fatty acids. One end of the soap molecule is ionic and the other end is non-ionic. These ends behave differently. The ionic end is hydrophilic and it is oriented towards water.
The other end is the hydrocarbon end, which is oriented towards dirt, which is oily in nature. When soap gets mixed with greasy dirt, micelles get formed around the oily dirt. When flushed with excess of water, the micelle containing the dirt is washed away and the fabric gets cleaned.
Fill In The Blanks
1. The element with atomic number 6 is carbon
2. Carbon has four valence electrons in its atom. The valency of carbon is four
3. Compounds formed due to the sharing of electron pairs between the participating atoms are known as covalent compounds
4. The property of carbon atom to form bonds with its own atoms is known as catenation
5. Water that does not give free lather with soap is called hard water
6. Organic compounds that have only a single bond between their carbon atoms are called saturated compounds
7. Organic compounds which have either a double bond or a triple bond between their carbon atoms are known as unsaturated compounds
8. Unsaturated hydrocarbons that have a double bond between their carbon atoms are called alkenes
9. The existence of two or more different organic compounds with the same molecular formula but different chemical and physical properties is called isomerism
10. The name of the alkene containing four carbon atoms is butene
![]()
Multiple Choice Questions
Question 1.
Ethane, with the molecular formula C2H6 has
(A) 6 covalent bonds
(B) 7 covalent bonds.
(C) 8 covalent bonds.
(D) 9 covalent bonds.
Answer:
(B) 7 covalent bonds.
Question 2.
Butanone is a four-carbon compound with the functional group
(A) carboxylic acid.
(B) aldehyde.
(C) ketone.
(D) alcohol.
Answer:
(C) ketone.
Question 3.
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that the
(A) food is not cooked completely.
(B) fuel is not burning completely.
(C) fuel is wet.
(D) fuel is burning completely.
Answer:
(B) fuel is not burning completely.
Question 4.
The number of single bonds in methane molecule is
(A) three
(B) four
(C) five
(D) six
Answer:
(B) four
Question 5.
Which of the following has -COOH as the functional group?
(A) Carboxylic acid
(B) Alcohol
(C) Aldehyde
(D) Ketone
Answer:
(A) Carboxylic acid
Question 6.
The minimum number of carbon atoms that should be present in a hydrocarbon for isomerism is
(A) 2
(B) 4
(C) 6
(D) 8
Answer:
(B) 4
Question 7.
Which of the following is a true statement about graphite and diamond?
(A) They have the same crystal structure.
(B) They have the same degree of hardness.
(C) They have the same electrical conductivity.
(D) They can undergo the same chemical reactions.
Answer:
(D) They can undergo the same chemical reactions.
Question 8.
The number of electrons in the outermost shell of carbon atom is
(A) 1
(B) 2
(C) 3
(D) 4
Answer:
(D) 4
Question 9.
The common name of ethanoic acid is
(A) Acetic acid
(B) Formic acid
(C) Propionic acid
(D) Butanoic acid
Answer:
(A) Acetic acid
![]()
Question 10.
The colour of the flame when the fuel burns in sufficient supply of oxygen is
(A) Yellow
(B) Blue
(C) Orange
(D) White
Answer:
(B) Blue
Question 11.
An example for an unsaturated hydrocarbon is
(A) cyclohexane
(B) butane
(C) isobutane
(D) benzene.
Answer:
(D) benzene.
Question 12.
In a double covalent bond, the number of electrons shared between the atoms is
(A) 2 electrons
(B) 3 electrons
(C) 4 electrons
(D) 6 electrons
Answer:
(C) 4 electrons
Question 13.
Covalent compounds are generally
(A) soluble in water
(B) insoluble in water
(C) ionize in water
(D) hydrolyse in water
Answer:
(B) insoluble in water
Question 14.
A salt that causes hardness in water is
(A) calcium sulphate
(B) magnesium bicorbonate
(C) calcium chloride
(D) Any of the above
Answer:
(D) Any of the above
Question 15.
Alkaline hydrolysis of an ester is known as
(A) esterification
(B) saponification
(C) hydration
(D) hardening
Answer:
(B) saponification
Question 16.
Two neighbouring compounds of a homologous series differ by
(A) CH
(B) CH2
(C) CH3
(D) CHO
Answer:
(B) CH2
![]()
Question 17.
The reaction in which an unsaturated hydrocarbon combines with another substance to give a single product is called
(A) Combustion reaction
(B) combination reaction
(C) Substitution reaction
(D) Addition reaction
Answer:
(D) Addition reaction
Question 18.
Vinegar is a solution of
(A) 50%-60% acetic acid in alcohol
(B) 5%-8% acetic acid in alcohol
(C) 5%-8% acetic acid in water
(D) 50%-60% acetic acid in water
Answer:
(C) 5%-8% acetic acid in water
Question 19.
Normal butane and iso-butane have
(A) the same molecular formula but different structural formula
(B) the same structural formula but different molecular formula
(C) the same molecular formula and the same structural formula
(D) different structural formula and different molecular formula
Answer:
(A) the same molecular formula but different structural formula
Question 20.
Which of the following statements concerning the members of a homologous series are correct?
(A) Each of them differs from the next one by a -CH2 group.
(B) They have the same functional group.
(C) They have the same chemical properties.
(D) All of the above are correct.
Answer:
(D) All of the above are correct.
Question 21.
In which of the following compounds, —OH is the functional group?
(A) Butanone
(B) Butanol
(C) Butanoic acid
(D) Butanal
Answer:
(B) Butanol
Question 22.
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
(A) Addition reaction
(B) Substitution reaction
(C) Displacement reaction
(D) Oxidation reaction
Answer:
(A) Addition reaction
Question 23.
The name of the compound CH3-CH2-CHO is
(A) Propanal
(B) Propanone
(C) Ethanol
(D) Ethanal
Answer:
(A) Propanal
![]()
Question 24.
Ethanol reacts with sodium and forms two products. These are
(A) sodium ethanoate and hydrogen
(B) sodium ethanoate and oxygen
(C) sodium ethoxide and hydrogen
(D) sodium ethoxide and oxygen
Answer:
(C) sodium ethoxide and hydrogen
Question 25.
A molecule that has a triple bond is
(A) hydrogen molecule
(B) oxygen molecule
(C) nitrogen molecule
(D) ammonia molecule
Answer:
(C) nitrogen molecule
Math The Following
Question 1.
| Column A | Column B |
| 1. Ethane | a. C6H6 |
| 2. Propene | b. C3H6 |
| 3. Butyne | c. C2H6 |
| 4. Benzene | d. C6H12 |
| 5. Cyclohexane | e. C4H6 |
| f. C4H8 |
Answer:
1 – c, 2 – b, 3 – e, 4 – a, 5 – d