Karnataka 2nd PUC Chemistry Question Bank Chapter 12 Aldehydes, Ketones and Carboxylic Acids
You can Download Chapter 12 Aldehydes, Ketones and Carboxylic Acids Questions and Answers, Notes, 2nd PUC Chemistry Question Bank with Answers Karnataka State Board Solutions help you to revise complete Syllabus and score more marks in your examinations.
2nd PUC Chemistry Aldehydes, Ketones and Carboxylic Acids NCERT Textbook Questions
Question 1.
What is meant by the following terms ? Give an example of the reaction in each case.
1. Cyanohydrin
2. Acetal
3. Semicarbazone
4. Aldol
5. Hemiacetal
6. Oxime
7. Ketal
8. Imine
9. 2,4-DNP-derivative
10. Schiff’s base
Answer:
1. Cyanohydrin: Compounds possessing hydroxyl and cyano groups on the same carbon atom, ie. gem-hydroxy nitrates are called cyanohydrin. These are produced by addition of HCN to aldehydes or ketones in weakly basic medium. ,
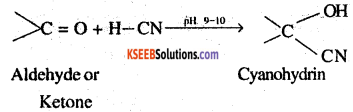
2. Acetal: Compounds in which the two alkoxy groups are present on the terminal carbon atom are called acetals. These are produced by the action of an aldehyde with two equivalents of a mono hydric alcohol in presence of dry HCl gas.
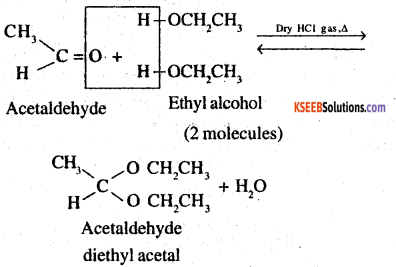
![]()
3. Semicarbazone: These are derivatives of aldehydes and ketones, and are produced by action of semicarbazide on them in weakly acidic medium.
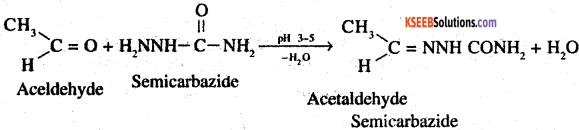
4. Aldol: Aldols are β- hydroxy aldehydes and are produced by the condensation of two molecules of the same or one molecule each of two different aldehydes in presence of a dilute aqueous base such as NaOH, KOH, Ba(OH)2 etc For eg:

5. Hemiacetal: They are gem-alkoxy alcohol. These are produced by addition of one molecule of a monohydric alcohol to an aldehyde in presence of dry HCl gas.

6. Oxime: Oximes are derivatives of aldehydes and ketones. These are produced when aldehydes or ketones react with hydroxy alamine in weakly acidic medium
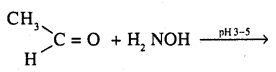
7. Ketal: Ketals are gem-dialkoxyalkanes.
In ketals, the two alkoxy groups are present on the same carbon within the chain, These are produced when a ketones is heated with ethylene glycol in the presence of dry HCl gas or p-toluene sulphonic acid (PTS).

8. Imine: Nucleophilic addition of ammonia to aliphatic aldehydes (except formaldehyde) in ether followed by dehydration gives an imine.
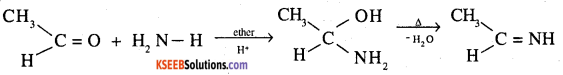
9. 2,4-DNP-derivative: These are produced when aldehyde or ketones react with 2,4 dinitrophenyl hydrazine in weakly acidic medium.
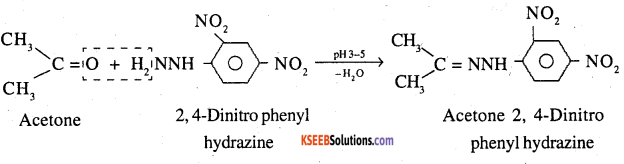
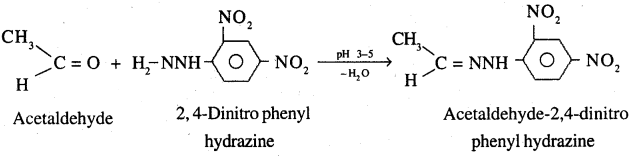
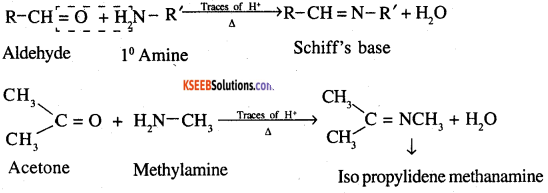
10. Schiff’s base: Aldehydes and ketones react with primary aliphatic or aromatic amines to form Schiff’s bases.
Question 2.
Name the following compounds according to IUPAC system of nomenclature:
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CH0-p
Answer:
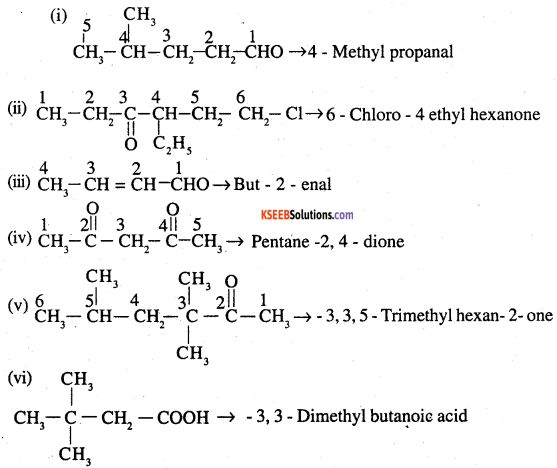
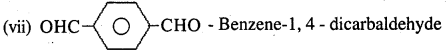
![]()
Question 3.
Draw the structures of the following compounds.
(i) 3-Methylbutanal
(ii) p-Nitropropiophenone
(iii) p-Methylbenzaldehyde
(iv) 4-Methylpent-3-en-2-one
(v) 4-Chloropentan-2-one
(vi) 3-Bromo-4-phenylpentanoic acid
(vii) p,p1-Dihydroxybenzophenone
(viii) Hex-2-en-4-ynoic acid
Answer:
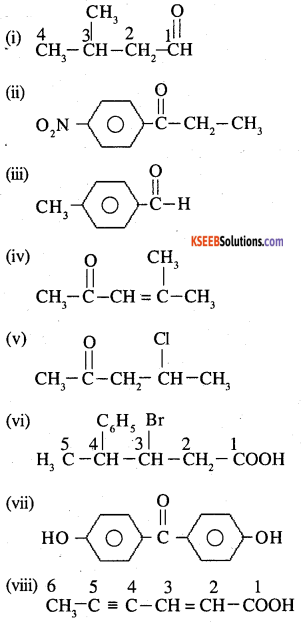
Question 4.
Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
(i) CH3CO(CH2)4CH3
(ii) CH3CH2CHBrCH2CH(CH3)CHO
(iii) CH3(CH2)5CHO
(v)
(iv) Ph-CH=CH-CHO
Answer:

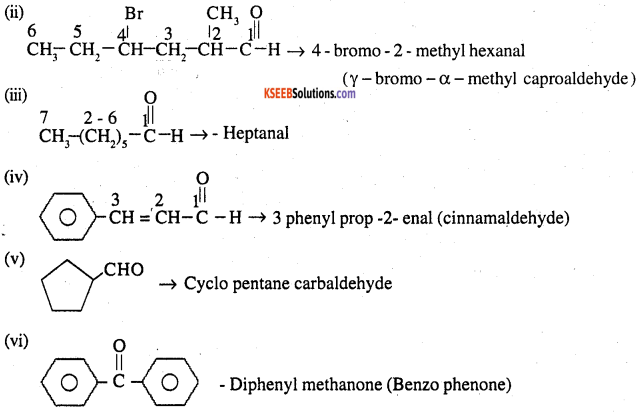
![]()
Question 5.
Draw structures of the following derivatives.
(i) 2,4-dinitrophenylhydrazone of benzaldehyde
(ii) Cyclopropanone oxime
(iii) Acetaldehydedimethylacetal
(iv) The semicarbazone of cyclobutanone
(v) The ethylene ketal of hexan-3-one
(vi) The methyl hemiacetai of formaldehyde
Answer:
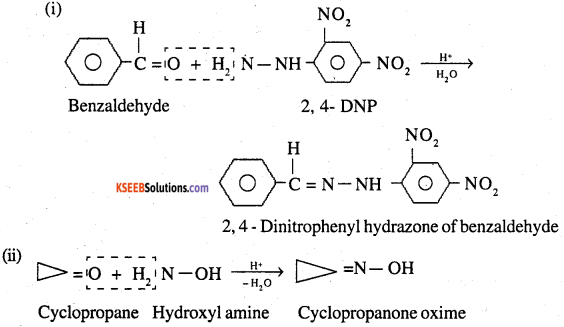
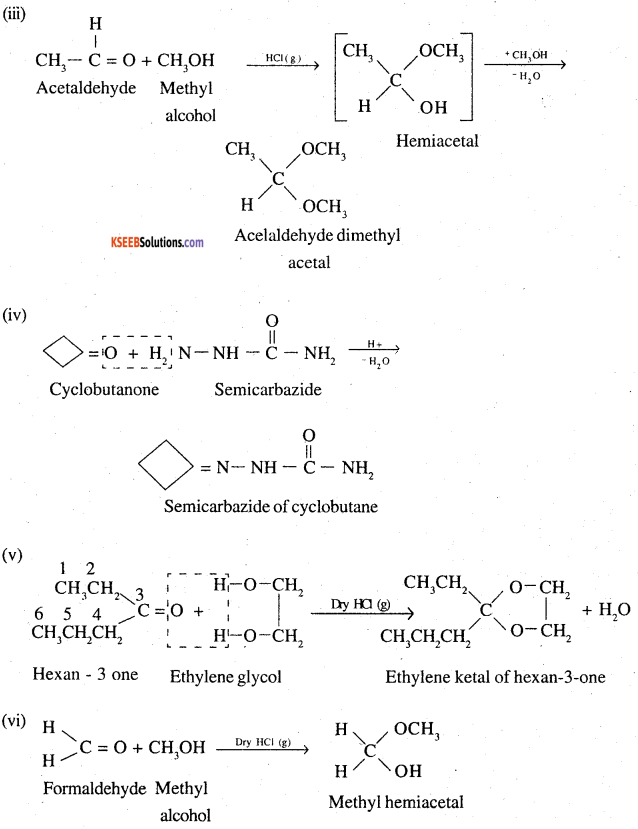
Question 6.
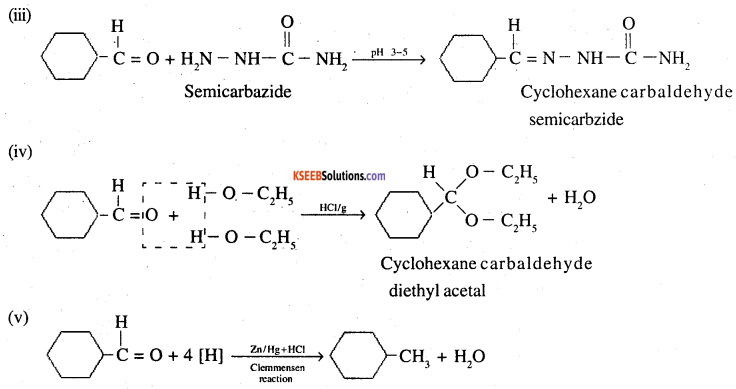
Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents.
(i) PhMgBr and then H3O+
(ii) Tollens’ reagent
(iii) Semicarbazide and weak acid
(iv) Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
Answer:
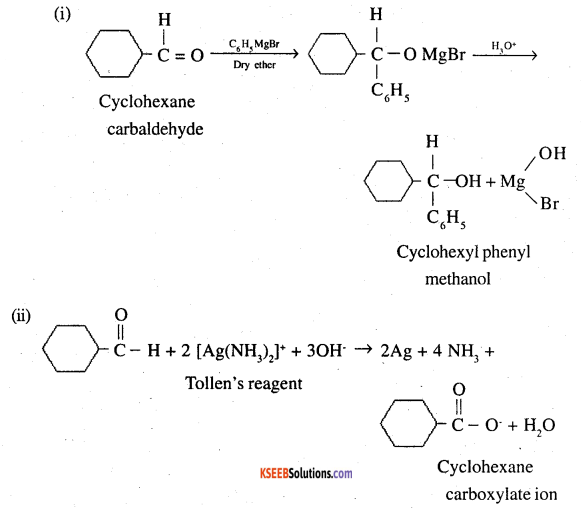
![]()
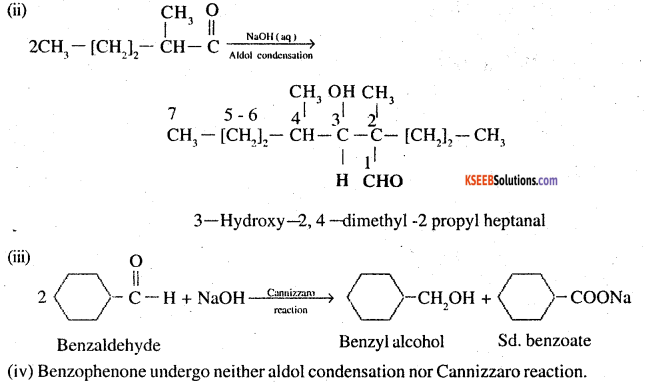
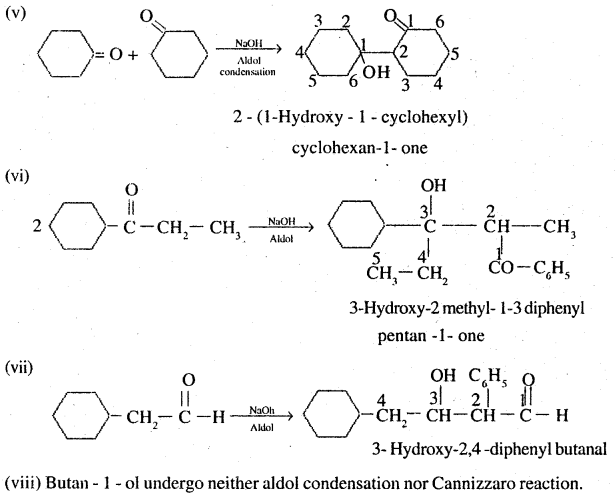
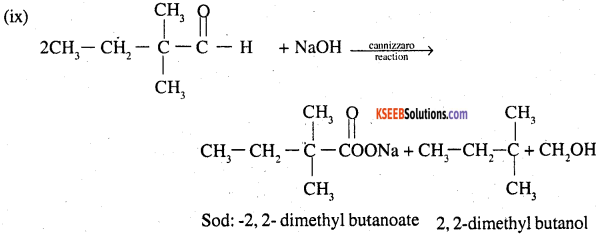
Question 7.
Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal
(ii) 2-Methylpentanar
(iii) Benzaldehyde
(iv) Benzophenone
(v) Cyclohexanone
(vi) l-Phenylpropanone
(vii) Phenylacetaldehyde
(viii) Butan-l-ol
(ix) 2,2-Dimethylbutanal
Answer:
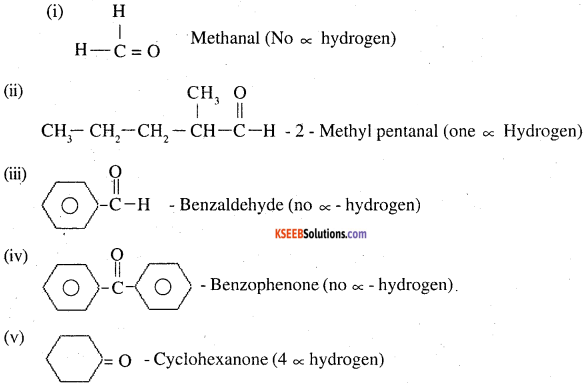
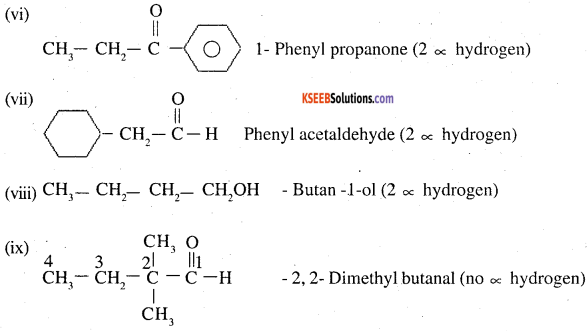
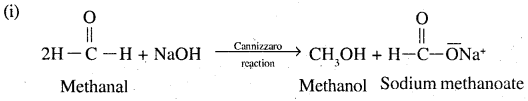
Thus ii, v, vii will undergo aldol condensation. Aldehydes i, iii, ix will undergo cannizzaro reaction. Ketones with no ∝ hydrogen iv. and vii, will undergo neither.
![]()
Question 8.
How will you convert ethanal into the following compounds?
(i) Butane-1,3-diol
(ii) But-2-enal
(iii) But-2-enoic acid
Answer:
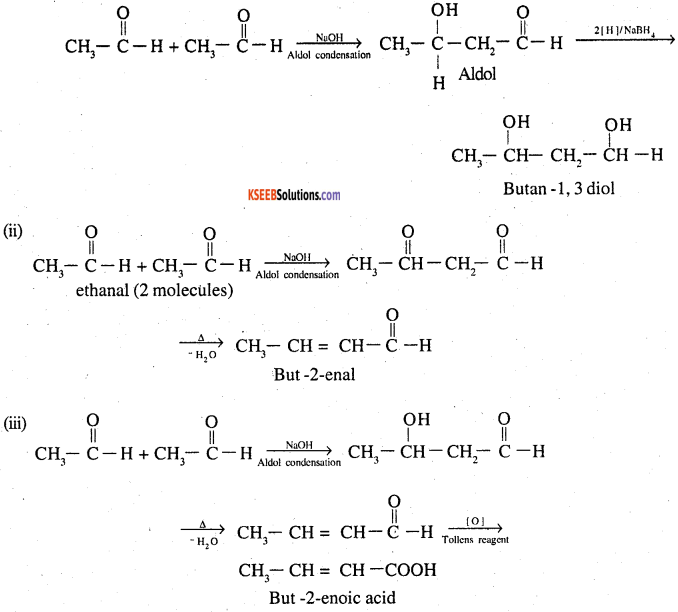
Question 9.
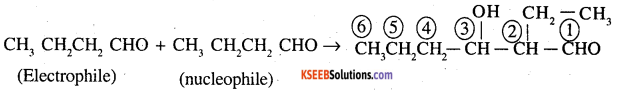
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Answer:
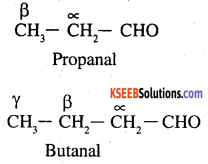
Both have 2 ∝ Hydrogen atom each. Therefore these can under go self as well as crossed aldol condensation 4 to give 4 different products.
(a) Propanal as nucleophile as well as electrophile
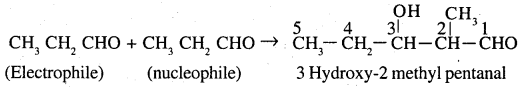
(b) Propanal as electrophile and butanal as nucleophile
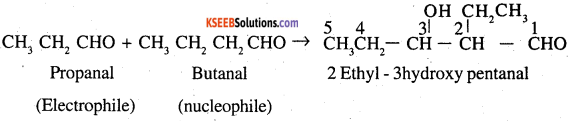
(c) Butanal as electrophile and propanal as nucleophile .
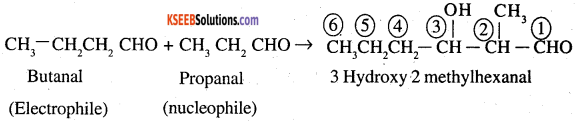
(d) Butanal both as nucleophile and electrophile
![]()
Question 10.
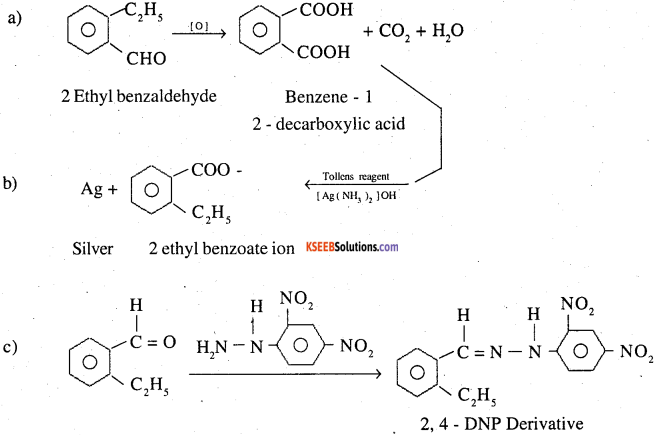
An organic compound with the molecular formula C9H10O forms 2,4-DNP derivative, reduces Tollens ’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
Answer:
1. The given organic compound forms 2, 4-DNP derivatives, therefore, it most be either an aldehyde or ketone.
2. The given organic compound reduces Tollens’ reagent. There fore it must be an aldehyde.
3. The given organic compound on oxidation gives 1,2- benzene dicarboxylic acid and it undergoes Cannizaro reaction. This indicates that it is an O substituted benzaldehyde.
4. Molecular formula of given organic compound is C9H10O. Therefore it is O-ethylbenzaldehyde or 2-ethylbenzaldehyde.
Question 11.
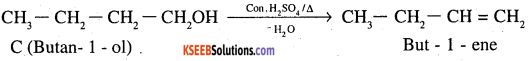
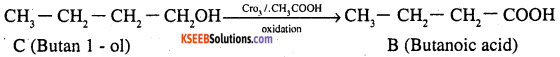
An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-l-ene.
Write equations for the reactions involved.
Answer:
1. Since A (M.F – C8H16O2) On hydrolysis gives a carboxylic acid B and an alcohol C, therefore A must be an ester.
2. Alcohol ‘C’ on oxidation giyes carboxylic acid B, this means that both B and C contains equal number of carbon atoms ie each of them contains 4 carbon atoms. This further indicates that alcohol ‘C’ is a 1° alcohol ie it is butan- l-ol.
3. ‘C’ on dehydration gives but-l-ene. This confirms that ‘C’ is butan – l-ol
4. As ‘C’ on oxidation gives B, this means B must be n-butanoic acid.
5. The ester A which gives butanoic acid (B) and butan – l-ol (C) must be butyl butanoate (A)
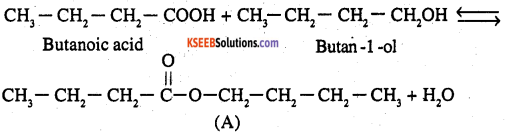
Question 12.
Arrange the following compounds in increasing order of their property as indicated:
1. Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
2. CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
3. Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength).
Answer:
1. Aldehydes and ketones undergo nucleophilic addition. In this case more the (ie. addition of HCN) +1 effect of the R-group and more the steric hindrace, lesser is the reactivity therefore, their increasing order of reactivity is
Di – tert – butyl ketone < Methyl tert butyl ketone < Acetone < Acetaldehyde.
2. In carboxylic acids +1 effect of R group decreases and -I effect of halogen increases the acidic character. Further, nearer the halogen atom to the carboxylic acid group, stronger is its -I effect. Therefore, the increasing order of acid strength is
(CH3)2CH-COOH < CH3-CH2– CH2– COOH < CH3– CH (Br) CH2COOH < CH3CH2-CH(Br) COOH
3. In benzoic acid and its derivatives, electron withdrawing group attached to ring increases its acidic strength while electron releasing group decreases the acidic strength. The effect is more pronounced if such a group is present at p-position.
Nitro group is electron withdrawing (‘R effect) while methoxy group in an electron releasing. There fore increasing order of acid strength is
4 methoxy benzoic acid < Benzoic acid
< 4 – Nitrobenzoic acid <3,4- Dinitro benzoic acid.
![]()
Question 13.
Give simple chemical tests to distinguish between the following pairs of compounds.
1. Propanal and Propanone
2. Acetophenone and Benzophenone
3. Phenol and Benzoic acid
4. Benzoic acid and Ethyl benzoate
5. Pentan-2-one and Pentan-3-one
6. Benzaldehyde and Acetophenone
7. Ethanal and Propanal
Answer:
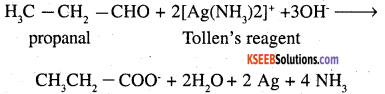
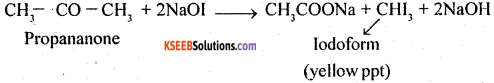
1. Propanal is an aldehyde and propanone is a ketone. These can be distinguished by
(a) Silver mirror test: Propanal reduces Tollens’ reagent to silver which propanone does not.
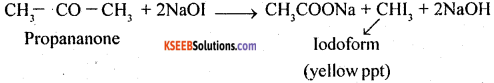
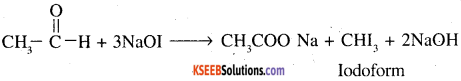
(b) Iodoform test: Propanone will give iodoform test while propanal will not give this test. Propanone gives yellow ppt of iodoform
2. Acetophenone and Benzophenone: Acetophenone contains CH3CO group, as such it will give iodoform test with NaOI (NaOH+I3) while no such group is there in benzophenone, so such it will not give iodoform test.
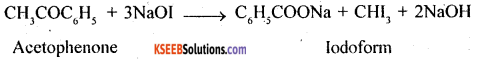
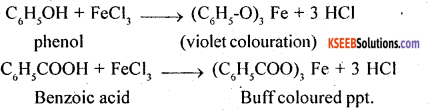
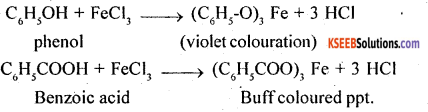
3. Phenol and Benzoic acid
(a) Sodium bicarbonate test: Benzoic acid is a stronger acid than carbonic acid (H2O + CO2), as such it will decompose sodium bicarbonate to give CO2. On the other hand, phenol is weaker acid than carbonic acid, as a result it will not decompose sodium bicarbonate.
C6H5COOH + NaHCO3 → C6H5COONa + CO2 + H2O
C6H5OH + NaHCO3 → No action
(b) Ferric chloride test: Phenol gives violet colouration with neutral FeCl3, solution while benzoic acid gives buff coloured ppt. of ferric benzoate.
4. Benzoic acid and Ethyl benzoate: Benzoic acid is an acid and ethyl benzoate is an ester. These may be distinguished by
(a) Sodium bicarbonate test: Benzoic acid will decompose sodium bicarbonate to give CO2 while ethyl benzoate will not.
C6H5COOH + NaHCO3 → C6H5COONa + CO2 + H2O
C6H5COOC2H5 + NaHCO3 → no reaction
(b) Litmus test: Benzoic acid term blue litmus to red, while ethyl benzoate wil l not.
5. Pentan-2-one and Pentan -3 -one
Pentan – 2-one contains CH3CO group and as such it will give iodo-form test with NaOI [NaOH + I2] while no such group is there in pentan -3- one and so it will not give iodoform test.
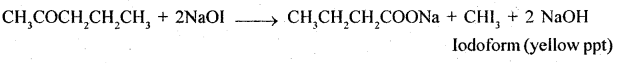
6. Benzaldehyde and Acetophenone
Benzaldehyde is an aromatic aldehyde while acetophenone is a methyl ketone. These may be distinguished as follows.
(a) Iodoform test: Acetophenone, due to the presence of CH3CO group, will give iodoform test with NaOI (NaOH +I2), while benzaldehyde will not give this test.

(b) Silver mirror test:- Benzaldehyde, being an aromatic aldehyde, will reduce tollens’ reagent to silver. On other hand acetophenone, a ketone will not give this test.
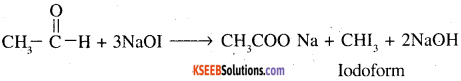
7. Ethanal and Propanal: As ethanal contains CH3CO group it will give iodoform test with NaOH and I2. On the other hand propanal will not give this test
Question 14.
How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom
1. Methyl benzoate
2. m-Nitrobenzoic acid
3. p-Nitrobenzoic acid
4. Phenylacetic acid
5. p-Nitrobenzaldehyde.
Answer:
1. Benzene to methyl benzoate
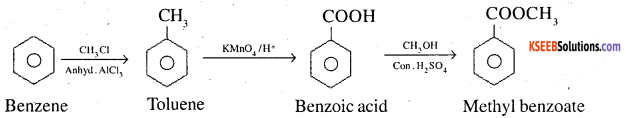
2. Benzene to m-Nitrobenzoic acid
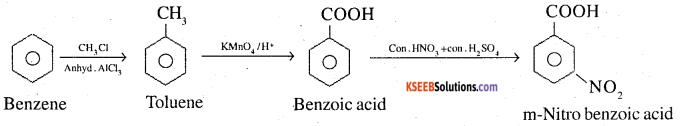
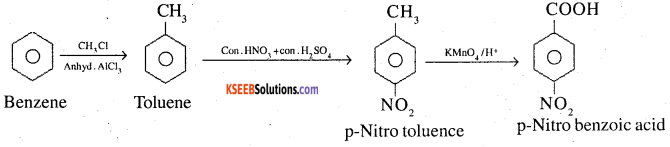
3. Benzene to p-Nitrobenzoic acid
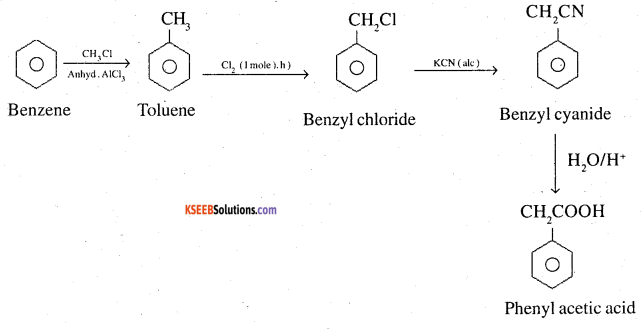
4. Benzene to phenyl acetic acid
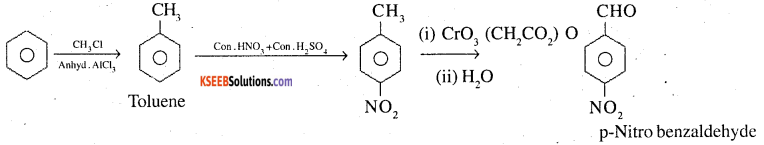
5. Benzene to p-nitro benzaldehyde
![]()
Question 15.
How will you bring about the following conversions in not more than two steps?
1. Propanone to Propene
2. Benzoic acid to Benzaldehyde
3. Ethanol to 3-Hydroxybutanal
4. Benzene to m-Nitroacetophenone
5. Benzaldehyde to Benzophenone
6. Bromobenzene to 1-Phenylethanol
7. Benzaldehyde to 3-Phenylpropan-l-ol
8. Benazaldehyde to a -Hydroxyphenylacetic acid
9. Benzoic acid to m- Nitrobenzyl alcohol
Answer:
1. Propanone to propene
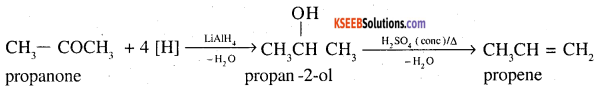
2. Benzoic acid to Benzaldehyde
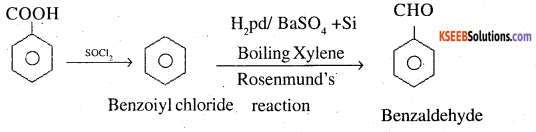
3. Ethanol to 3-Hydroxybutanal
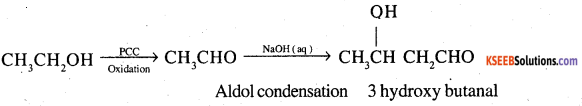
4. Benzene to m-Nitroacetophenone
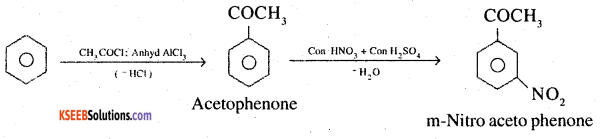
5. Benzaldehyde to benzophenone
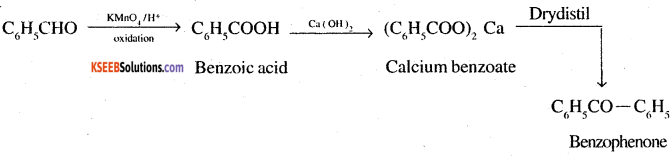
6. Bromobenzene to 1-Phenylethanol
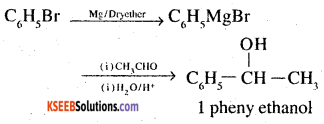
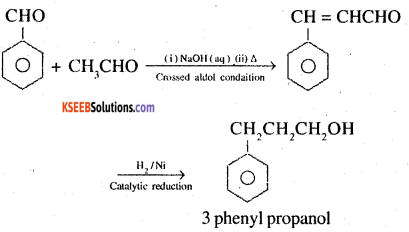
7. Benzaldehyde to 3-Phenylpropan-1 -ol
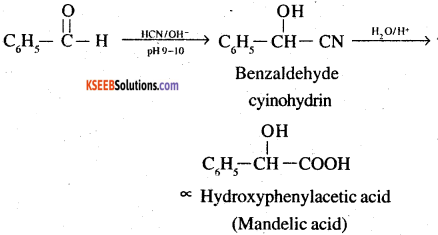
8. Benazaldehyde to a -Hydroxyphenylacetic acid O OH
9. Benzoic acid to m- Nitrobenzyl alcohol
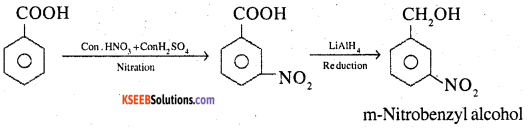
![]()
Question 16.
Describe the following:
1. Acetylation
2. Cannizzaro reaction
3. Cross aldol condensation
4. Decarboxylation
Answer:
1. Acetylation: The replacement of active hydrogen of alcohols, phenols, amines or even benzene with acetyl group (CH3CO-) is called acetylation. This is always carried out with acetoyl chloride, or acetic anhydride
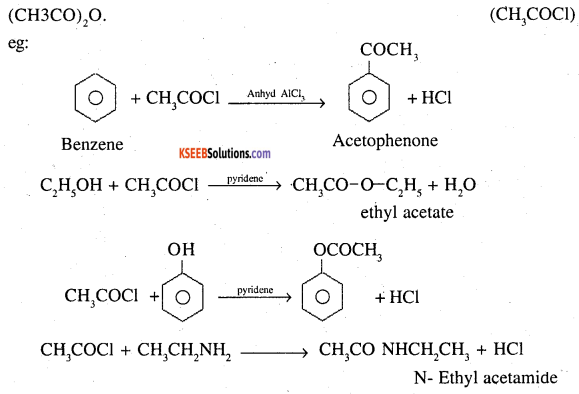
2. Cannizzaro reaction: Aldehydes which do not contain an oc hydroges when treated with concentrated solution of an alkali undergo self oxidation reduction. As a result one molecule of aldehyde is reduced to corresponding alcohol while the other molecule is oxidised to the corresponding acid. This reaction is called Cannizzaro reaction. Aldehydes such as formaldehyde, benzaldehyde (C6H5CHO) which do not contain ∝ hydrogen undergo this reaction.
3. Cross aldol condensation: Condensation between 2 defferent aldehydes or ketones, at least one of which contains ∝ – hydrogen is called aldol condensation. It is a useful synthetic tool only when one of the aldehyde or ketone does not contains ∝ – hydrogen. This is because if both the aldehydes or ketones contain ∝ – hydrogen it gives a mixture of four different products.
eg:- Condensation between formaldehyde and acetaldehyde in the presence of dil NaOH (aq)
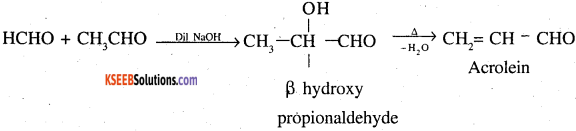
4. Decarboxylation:- The process of removal of CO2 from a carboxylic acid is called decarboxylation. It is generally carried out by heating the acid or its sodium salt with soda lime (NaOH + CaO) at 630 K.
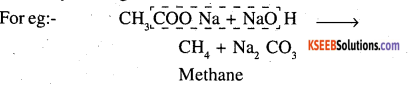
Question 17.
Complete each synthesis by giving missing starting material, reagent or products
Answer:
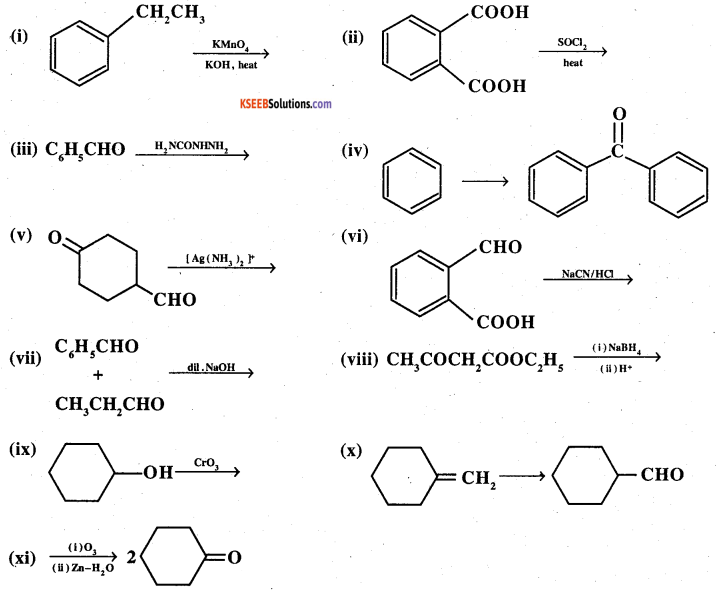
Answer:
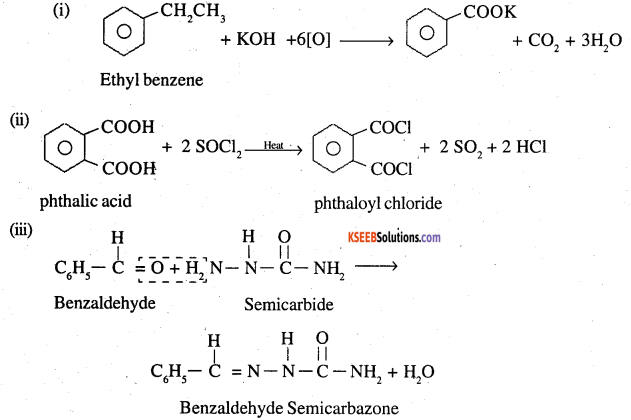
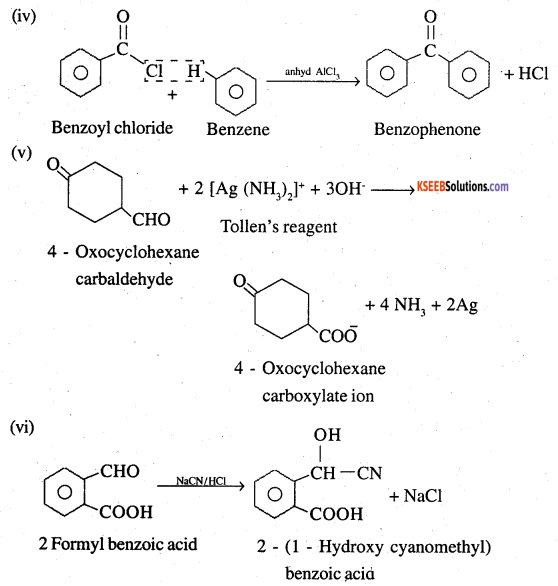
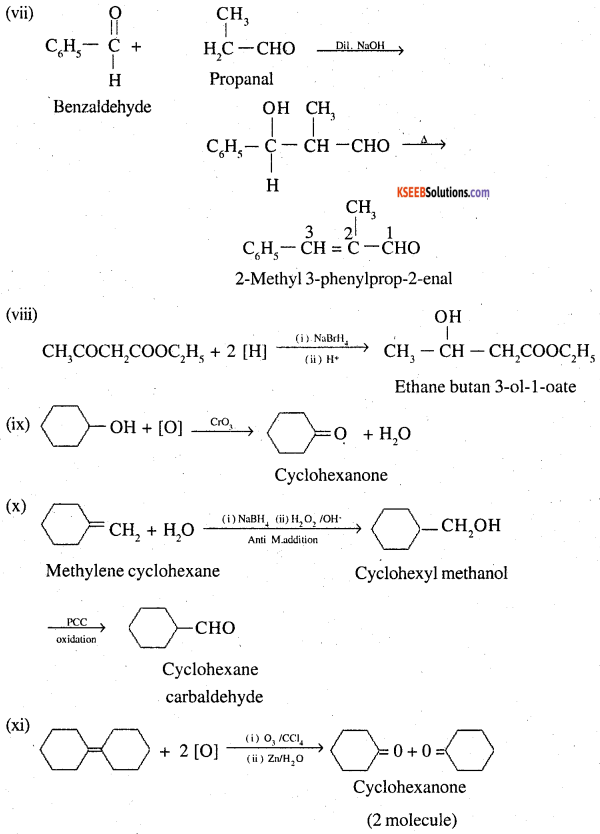
![]()
Question 18.
Give plausible explanation for each of the following:
1. Cyclohexanone forms cyanohydrin in good yield but 2,2,6-trimethylcyclohexanone does not.
2. There are two -NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
3. During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Answer:
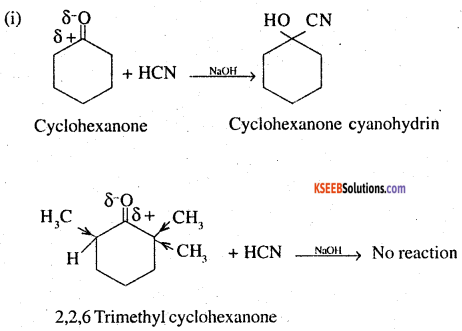
This is due to steric hindrance and +1 effect of three methyl groups in 2,2,6 – trimethyl cyclohexane. Addition of HCN to >C = O group is an example of nucleophile addition in which the nucleophile ie :CN attacks the carbon of carbonyl group. The presence of 3 methyl groups at ∝ position w.r.t. >C = O group leads to steric hindrance and reduction of positive charge on the carbonyl carbon,
2. Semicarbide is resonance hybrid of following structures.
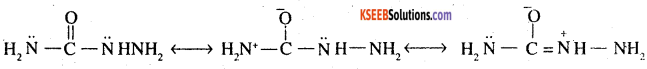
Although semicarbide has 2 -NH2 groups but one of thefn (i.e. which is directly attached to >C = O group) is involved in resonance as shown. As a result, electron density on this NH2 group decreases and hence it does not act as a nucleophile. In contrast, the lone pair of e’ on the other NH2 group (i.e. attached to NH) is not involved in resonance and hence is available for nucleophilic attack on carbon of the carbonyl group of aldehydes and ketones.
3. The formation of the esters from a carboxylic acid and an alcohol in presence of an acid catalyst is a reversible reaction
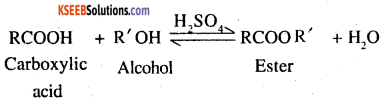
To shift the equilibrium in the forward direction, the water or the ester formed (which ever is more volatile) should be removed as fast as it is formed.
Question 19.
An organic compound contains 69.77% carbon, 11,63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. On vig¬orous oxidation it gives ethanoic and propanoie acid. Write the possible structure of the compound.
Answer:
First we find out the molecular formula of the compound
% of O2 = 100 – (% of C + % of H) = 100 – (69.77 + 11.63)
= 100 – (81.40)= 19.6%
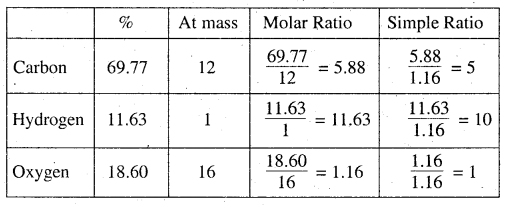
Empirical formula of the compound = C5H10O
Empirical formula mass = 5 × 12 + 10 × 1 + 16 × 1 = 86
but given that molecular mass = 86
![]()
∴ molecular formula of the compound (C5H10O),
= C5H10O
Now we will find the structure of the compound
It is given that
1. It does not reduce Tollens’ reagent but forms addition compound with sodium hydrogen sulphide. As we know that only methyl ketone and aldehydes react with sodium hydrogen sulphide, the compound is methyl ketone. Also given that it given iodoform test. Thus the given compound can be represented as
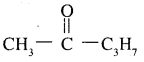
2. On vigorous oxidation it gives ethanoic acid and propanoic acid. This shows that the propyl group attached to ![]() group in n-propyl and not isopropyl. So the compound is
group in n-propyl and not isopropyl. So the compound is
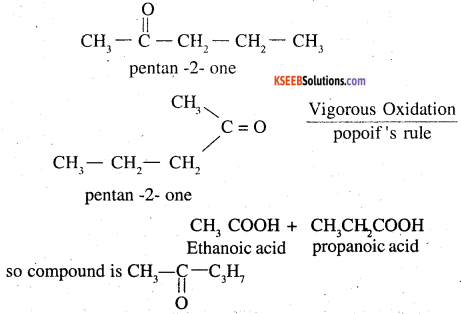
Question 20.
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Answer:
Both aliphatic carboxylic acid (R COOH) and phenol have tendency to release protons as follows
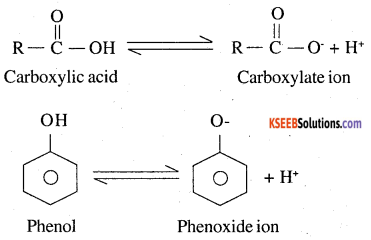
Both carboxylate ion and phenoxide ion are resonance stabilised as explained :
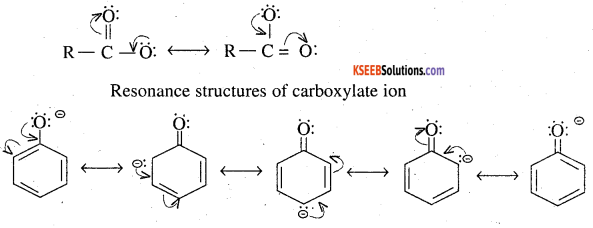
Although phenoxide ion has more number of resonating structure (five) than carboxylate ion (two), yet carboxylate ion is better resonance stabilised than phenoxide ion. It is due to the following two facts.
1. The two contributing structure of carboxylate ion are structurally equivalent, but not in the case of phenomide ion
2. In carboxylate ion the negative charge is dispersed over two oxygen atoms, where as in phenoxide ion, in three out of five resonance structure, the negative charge is less on less electronegative carbon atom.
Due to better resonance stabilisation of carboxylate ion the equilibrium is shifted more towards the right hand side incase of carboxylic acid than in phenol. Therefore carboxylic acid have a greater tendency to release the proton. That is carboxylic acid is more acidic than phenol.
![]()
Question 21.
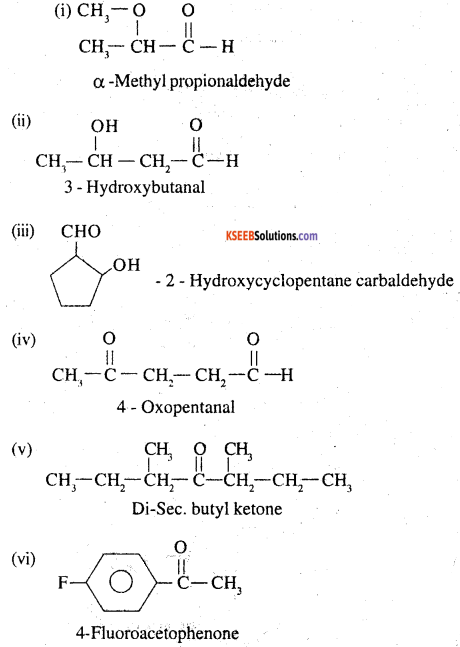
Write the structures of the following compounds.
(i) α -Methoxypropionaldehyde
(ii) 3-HydroxybutanaI
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec. butyl ketone
(vi) 4-Fluoroacetophenone
Answer:
Question 22.
Write the structures of products of the following reactions;
(i)
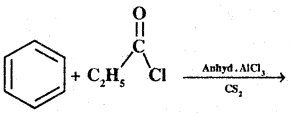
(ii) (C6H5CH2)2 Cd + 2 CH3COC1 →
(iii)
![]()
(iv)
Answer:
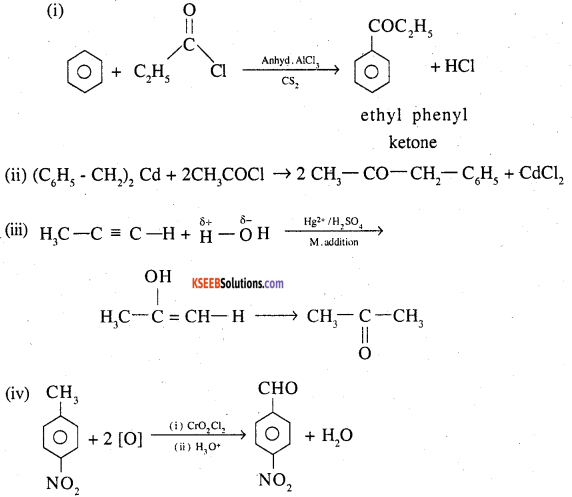
Question 23.
Arrange the following compounds in increasing order of their boiling points.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
Answer:
All the compounds have nearly the same atomic masses. Out of all these compounds propane is non polar and will have, as such, lowest boiling point. Dimethyl ether is polar and as such will have a higher boiling point than propane. Ethanal, more polar than dimethyl ether will have a higher boiling point than dimethyl ether. Ethanol will have the highest boiling point due tp Hydrogen bonding. There for increasing order of boiling point is
CH3 – CH2 – CH3 < CH3OCH3 < CH3CHO < CH3CH2OH.
![]()
Question 24.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
1. Ethanal, Propanal, Propanone, Butanne.
2. Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Hint: Consider steric effect and electronic effect.
Answer:
1. Butanne < Propanone < Propanal < ethanal
2. Acetophenone < p-Tolualdehyde < Benzaldehyde < p-Nitrobenzaldehyde
Question 25.
Predict the product of the following reactions:
Answer:
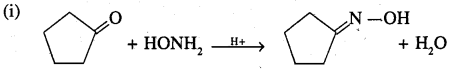
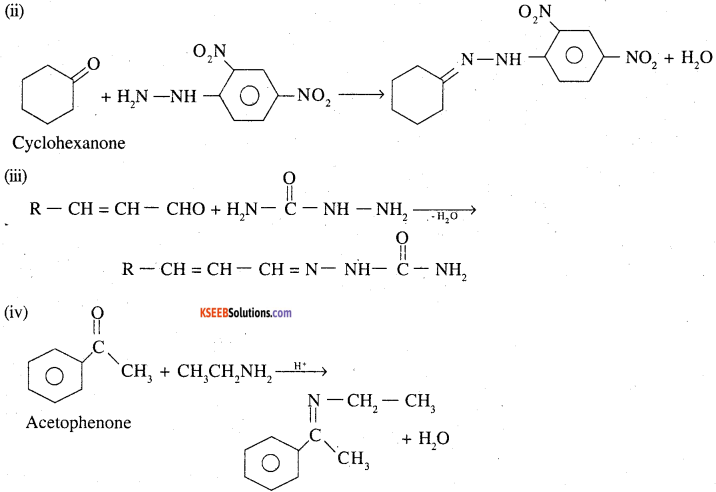
Question 26.
Give the IUPAC names of the following compounds:

Answer:
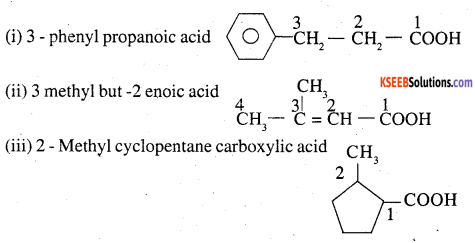
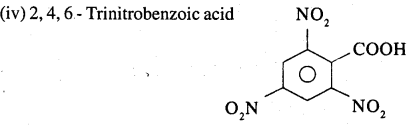
Question 27.
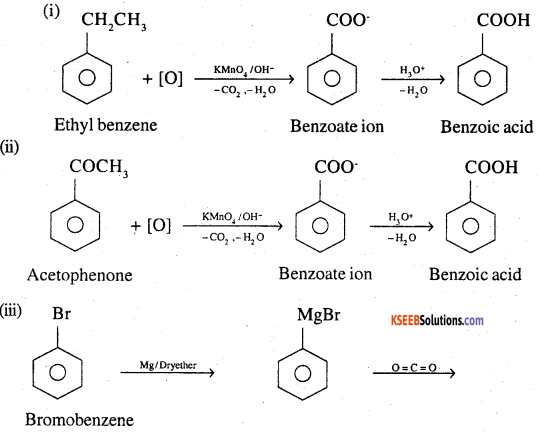
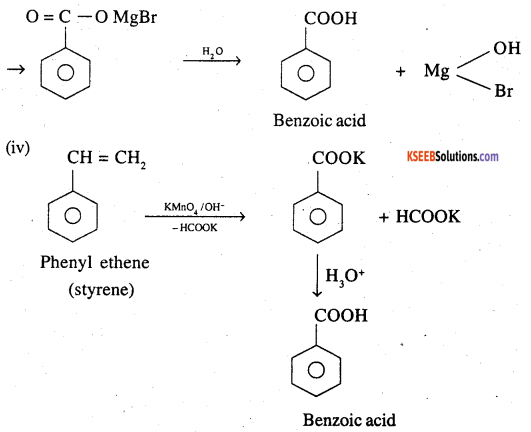
Show how each of the following compounds can be converted to benzoic acid,
(i) Ethylbenzene
(ii) Acetophenone
(iii) Bromobenzene
(iv) Phenylethene (Styrene)
Answer:
Question 28.
Which acid of each pair shown here would you expect to be stronger?
(i) CH3,CO2H or CH2FCO2H
(ii) CH2FCO2H or CH2ClCO2H
(iii) CH2FCH2CH2CO2H or CH3CHFCH2CO2H
(iv)
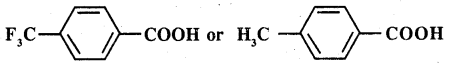
Answer:
We know that atoms or groups which decrease the electron density on C-atom of -COOH group make the acid stronger while atoms or groups which increases the electron density at C-atom of -COOH group make the acid weaker.
1. Due to -I effect of F-atom, FCH2-COOH is a stronger acid than CH3-COOH
2. Due to stronger -I effect of F-atom than Cl atom, FCH2COOH is a stronger acid than ClCH2COOH.
3. Due to -I effect of fluorine atom at β -position than at γ -position, CH3 – CH (F) CH2-COOH is stronger than CH2(F) CH2CH2COOH.
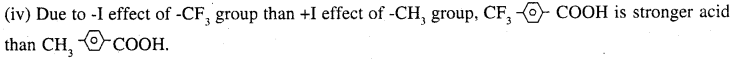
![]()
2nd PUC Aldehydes, Ketones and Carboxylic Acids Additional Questions
2nd PUC Aldehydes, Ketones and Carboxylic Acids Two Marks Questions and Answers
Question 1.
Mention 2 important uses of formaline? (CBSE 1997,2000)
Answer:
- As a preservation for biological samples
- It is used in the manufacture of thermo setting plastics.
Question 2.
What product is obtained when ethyl benzene is oxidised with alkaline KMnO4 ? (CBSE 2001)
Answer:
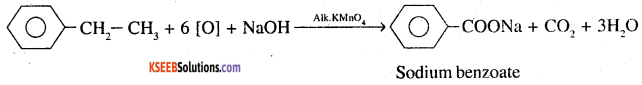
Question 3.
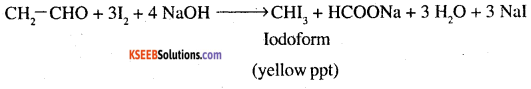
Give the chemical test to distinguish between acetaldehyde and benzaldehyde. (CBSE 2002)
Answer:
Acetaldehyde only gives iodoform test
Question 4.
Write the chemical test to distinguish between formic acid and acetic acid
Answer:
Only formic acid gives Tollens’ reagent test

Question 5.
Mention an industrial product manufactured from methanal. (AISB 2002)
Answer:
Bakelite.
Question 6.
Write the uses of methanoic acid.
Answer:
It is used in rubber industry for coagulating latex, it is also used in textile, dyeing, leather and electroplating industries.
Question 7.
Write the IUPAC names of (DSB 2003, 2004)
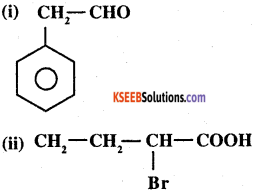
Answer:
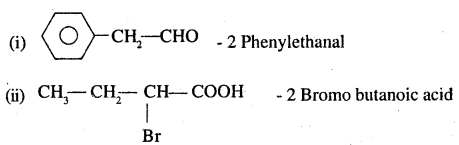
Question 8.
Draw the structure of 4 methyl pent -3 ene-2one. (AISB 2004,2005)
Answer:
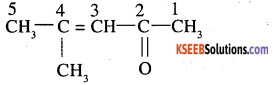
Question 9.
Write the structural formula of the following compounds (AISB 2005, 2006)
(i) 3-Bromo-4-phenyl pentanoic acid
(ii) Hex -2-en-4- ynoic acid
Answer:
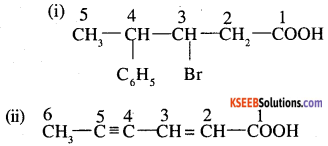
![]()
2nd PUC Aldehydes, Ketones and Carboxylic Acids Three Marks Questions and Answers
Question 1.
Write the equations and conditions to show how the following conversion are carried out
(i) Benzaldehyde into acetophenone. (CBSE 1995,1999)
(ii) Malonic acid into acetic acid (DSB 1999)
(iii) Acetaldehyde into but 2 enal (DSB 2000)
Answer:
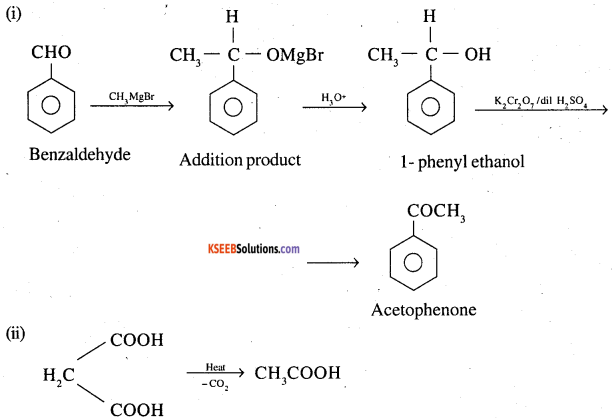
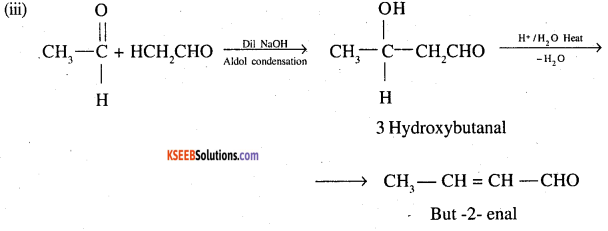
Question 2.
Give explanation for the following observation:
Towards nucleophile regents, aldehydes are more reactive than ketones (DSB 2000, 2006)
Answer: This is due to 2 reasons
1. Inductive effect
Alkyl group is an electron releasing groups (+1 inductive effect). Therefore, electron releasing power of two alkyl groups in ketones is more than that of one in aldehyde. As a result, the electron deficiency of carbon atom in the carbonyl group is satisfied more in ketones than in aldehydes. Therefore, the reduced positive charge on carbon in case of ketones discourages the attack of nucleophiles. Hence ketones are less reaction than aldehydes.
2. Stearic effect
The size of alkyl group is more than that of Hydrogen. In aldehydes only one alkyl group but in ketones 2 alkyl groups are attached to the carboxyl group.
The alkyl groups are larger than a hydrogen atom and these causes hindrance to the attacking group.
This is called stearic effect.
As the number and size of the alkyl group increase, the hindrance to the attack of nucleophile also increases and reactivity decreases.
Question 3.
How would you obtain the following from the named sources
(i) Tertiary butyl alcohol from acetone
(ii) Acetone from acetic acid
(iii) Benzene to acetophenone
Answer:
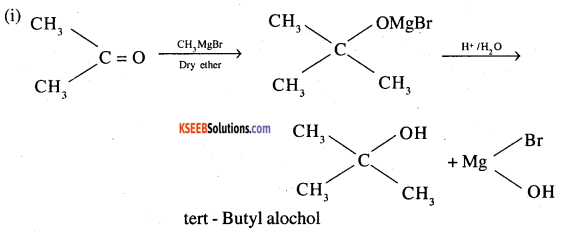
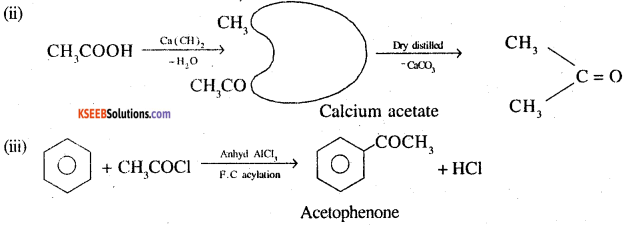
Question 4.
State one chemical method each to distinguish between the following pairs of organic compounds
1. Acetaldehyde and acetone (CBSE 1996,2001,2002, DSB 2000, 2001, 2003)
2. Phenol and benzoic acid (CSBE – All India 2004)
3. Propanone and ethanol (DSB 2001, 2003)
Answer:
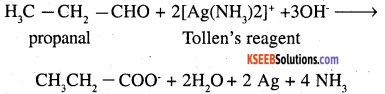
1. Acetaldehyde gives silver mirror test with Tollens’ regent where as acetone does not.
CH3 – CHO + 2 [Ag (NH3)2]+ OH →
CH3CHOONH4 + 2 Ag + 3NH3 + H2O
2. Treat each compound with sodium carbonate solution. Benzoic acid decomposes NaHCO, to evolve CO2, but phenol does not.
C6H5COOH + NaHCO3 → C6H5COONa + CO2 ↑+ H2O
3. Treat each compound with 2,4- DNP reagent. Only propanone gives an orange colour ppt
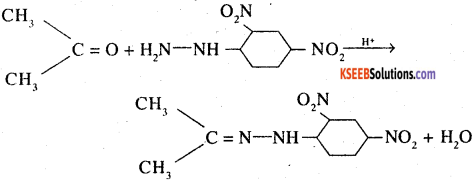
![]()
Question 5.
State one reaction as an example in each case
1. Rosenmund Reduction (Delhi 2001)
2. Cannizaro’s reaction (CBSE 1997, 2001, 2008)
Answer:
1.
![]()
2. 2 HCHO + NaOH → CH3OH + HCOONa.
Question 6.
Describe the preparation of acetic acid from acetylene (CBSE 2003)
Answer:
Acetylene when treated with 42% H2SO4 and 1% of HgSO4 at 333K gives ethanoic acid
![]()
Question 7.
Why carboxylic acid do not give the characteristic reactions of carbonyl group ? (AISB 2003)
Answer:
Due to the presence of lone pairs of electrons on the oxygen atom of the OH group, the carboxylic acids may be regarded as resonance hybrid of the following
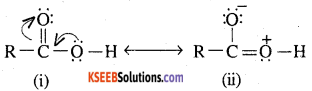
Similarly a carbonyl group of aldehydes and ketones may be regarded as resonance hybrid of the following structures
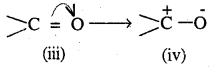
Due to the contribution of structures, (iii) & (iv), the carboxyl carbon in aldehydes and ketones is electrophilic. How ever, due to the contribution of structure (ii), the electrophilic character of carboxyl carbon is reduced.
Question 8.
Mention a chemical properly in which methanoic acid differs from acetic acid. (AISB 2005)
Answer:
Methanoic acid acts as reducing agent and hence decolourises the pink colour of acidified KMnO4 solution but acetic acid does not.

Question 9.
Give chemical test to distinguish between the following pairs of compounds (AISB 2005, 2006)
1. Propanoylchloride and Propanoic acid
2. Benzaldehyde and Acetophenone
Answer:
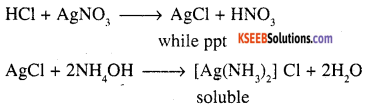
1. Propanoylchloride undergoes hydrolysis to give white fumes of HC1, which will give a dense white fumes of NH4Cl with NH3
CH3 – CH2COCI + H2O —> CH3CH2COOH + HCl
The products of hydrolysis will give a white ppt. of AgCl which is soluble in excess NH4OH
2.
1. Propanal is an aldehyde and propanone is a ketone. These can be distinguished by
(a) Silver mirror test: Propanal reduces Tollens’ reagent to silver which propanone does not.
(b) Iodoform test: Propanone will give iodoform test while propanal will not give this test. Propanone gives yellow ppt of iodoform
2. Acetophenone and Benzophenone: Acetophenone contains CH3CO group, as such it will give iodoform test with NaOI (NaOH+I3) while no such group is there in benzophenone, so such it will not give iodoform test.
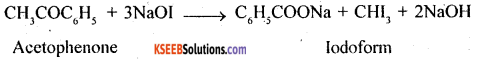
3. Phenol and Benzoic acid
(a) Sodium bicarbonate test: Benzoic acid is a stronger acid than carbonic acid (H2O + CO2), as such it will decompose sodium bicarbonate to give CO2. On the other hand, phenol is weaker acid than carbonic acid, as a result it will not decompose sodium bicarbonate.
C6H5COOH + NaHCO3 → C6H5COONa + CO2 + H2O
C6H5OH + NaHCO3 → No action
(b) Ferric chloride test: Phenol gives violet colouration with neutral FeCl3, solution while benzoic acid gives buff coloured ppt. of ferric benzoate.
4. Benzoic acid and Ethyl benzoate: Benzoic acid is an acid and ethyl benzoate is an ester. These may be distinguished by
(a) Sodium bicarbonate test: Benzoic acid will decompose sodium bicarbonate to give CO2 while ethyl benzoate will not.
C6H5COOH + NaHCO3 → C6H5COONa + CO2 + H2O
C6H5COOC2H5 + NaHCO3 → no reaction
(b) Litmus test: Benzoic acid term blue litmus to red, while ethyl benzoate wil l not.
5. Pentan-2-one and Pentan -3 -one
Pentan – 2-one contains CH3CO group and as such it will give iodo-form test with NaOI [NaOH + I2] while no such group is there in pentan -3- one and so it will not give iodoform test.
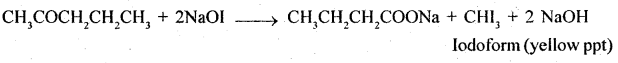
![]()
6. Benzaldehyde and Acetophenone
Benzaldehyde is an aromatic aldehyde while acetophenone is a methyl ketone. These may be distinguished as follows.
(a) Iodoform test: Acetophenone, due to the presence of CH3CO group, will give iodoform test with NaOI (NaOH +I2), while benzaldehyde will not give this test.

(b) Silver mirror test:- Benzaldehyde, being an aromatic aldehyde, will reduce tollens’ reagent to silver. On other hand acetophenone, a ketone will not give this test.
7. Ethanal and Propanal: As ethanal contains CH3CO group it will give iodoform test with NaOH and I2. On the other hand propanal will not give this test
Question 10.
Name the reagent you will use to bring about the following conversions
(a) Ethane nitrite to ethanal
(b) Alkyl alcohol to propanal
(c) But- 2-ene to ethanal
Answer:
(a) (Diisobutyl) aluminium hydride (DIBAL-H)
(b) Pyridinium chlorochromate (PCC)
(c) (i) O3/CCl4 (ii) ZnDust /H2O
Question 11.
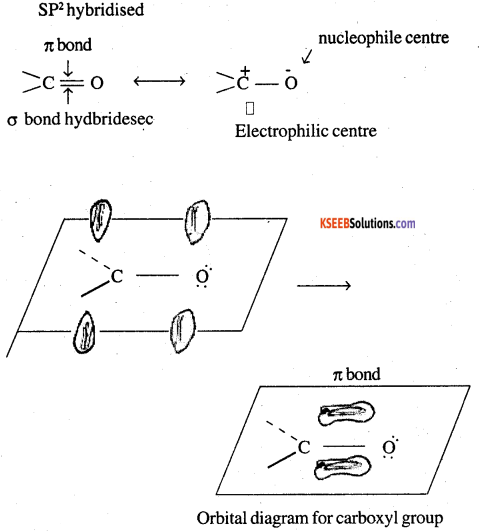
Draw the structure of a carbonyl group and indicate clearly
(i) the hybridesed state of carbon
(ii) the a and n bonds!’present and
(iii) the electrophilic and nucleophile centres in it.
Answer:
![]()