Students can Download 2nd PUC Chemistry Previous Year Question Paper March 2019, Karnataka 2nd PUC Chemistry Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka 2nd PUC Chemistry Previous Year Question Paper March 2019
Time: 3 Hrs 15 Min
Max. Marks: 70
Part-A
I. Answer all the following: (10 × 1 = 10)
Question 1.
How does the size of blood cells change when placed in an aqueous solution containing more than 0.9% (m/v) sodium chloride?
Answer:
Blood cells shrinks.
Question 2.
How does the volume change on mixing two volatile liquids to form an ideal solution?
Answer:
There is no change in the volume of the solution.
Question 3.
Draw a graph of Δm v/s \(\sqrt{\mathbf{C}}\) for acetic acid (weak electrolyte) solution.
Answer:
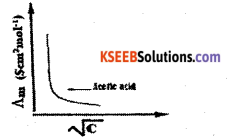
Question 4.
Unit of rate constant of a reaction is same as that of its rate. What is the order of this reaction?
Answer:
Zero order
![]()
Question 5.
Among physisorption or chemisorption which one has higher enthalpy of adsorption?
Answer:
Chemisorption
Question 6.
What is the role of depressant (NaCN) in Froth-Flotation method?
Answer:
It prevents froth formation by one sulphide ore and allowing the other to come into froth.
OR
If the ore contains mixture of ZnS and PbS, NaCN allows PbS to come with froth and ZnS to sink.
OR
It helps to separate two sulphide ores in a mixture like Zns and PbS.
Question 7.
Name the noble gas having ms2 np6 electronic configuration but does not have d-orbitals in its valence shell.
Answer:
Neon
Question 8.
Write the general equation for Wurtz reaction.
Answer:
R – X + 2Na + X – R → R – R + 2NaX
Question 9.
What is the reagent ‘A’ used in the following equation?
![]()
Answer:
LiAlH4
Question 10.
Which vitamin deficiency causes the diseases pernicious anaemia?
Answer:
Vitamin – B12.
Part-B
II. Answer any FIVE questions: (Each question carries two marks) (5 x 2=10)
Question 11.
Lithium metal has a body centre cubic lattice structure with edge length of unit cell 352 pm. Calculate the density of lithium metal.
[Given : Atomic mass of Li = 7 g mol-1 NA = 6.022 × 1o23 Atoms mol-1].
Answer:
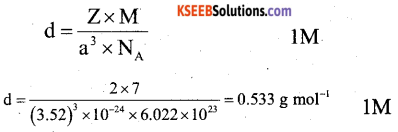
Question 12.
State Faraday’s second law of electrolysis.
Answer:
If same quantity of electric current is passed through different electrolyte solutions, the amount of substances discharged are proportional to their equivalent masses.
![]()
Question 13.
What is pseudo-first order reaction? Give an example.
Answer:
Higher order reactions are converted into first order reactions by taking all the reactants in large excess except one are called Pseudo first order reactions.
Example :
(i) Acid hydrolysis of methyl acetate
![]()
Rate = A[CH3COOCH3]1 [H2O]0 ∴ Order = 1 + 0 = 1
(ii) Hydrolysis of sucrose or inversion of cane sugar
![]()
In this reaction r = k[C12H22O11]1 [H2O]0 ∴ Order =1 + 0 = 1
Question 14.
How will you account for the following?
(a) Actinoids exhibit more number of oxidation states than lanthanoides.
(b) Atomic radii of second and third transition series elements are almost identical.
Answer:
(a) As in actinoids the outer electrons are less firmly held, they are easily available for bonding.
(b) due to lanthonoid contraction.
Question 15.
Explain the Kolbe’s reaction with equation.
Answer:
When sodium phenate is heated with carbon dioxide to 140°C under 6-7 atm pressure, sodium salicylate is obtained which on acidification gives salicylic acid.
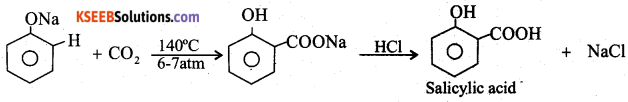
Question 16.
Write the equation for the reaction between benzaldehyde and concentrated NaOH solution. Name the reaction.
Answer:
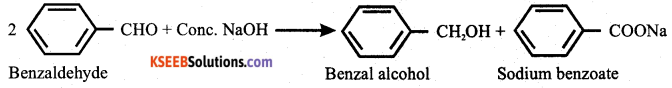
It is a Cannizzaro’s reaction.
Question 17.
(i) What are anionic detergents?
(ii) What is the role of saccharin in food?
Answer:
i) In anionic detergents large part of the molecules is anion and it is the anionic part of the molecule which is involved in their cleansing action.
ii) Artificial Sweetner.
Question 18.
Give one example each for the following.
(i) Antifertility drugs
(ii) Narcotic analgesics.
Answer:
(i) The chemicals which prevent the pregnancy in women are called anti-fertility drugs. Example: Mixture of norethindrone and ethynylestradiol (novestrol).
(ii) They produce sleep so they are used for the relief of post operative pain, cardiac pain, labour pain in child birth etc. Example: Morphine, heroin, codeine etc.
![]()
Part-C
III. Answer any FIVE of the following: (Each question carries three marks) (5 × 3 = 15)
Question 19.
(a) In the extraction of Aluminium by electrolysis.
(i) Write overall cell reaction.
Answer:
2Al2O3 + 3C → 4A1 + 3CO2
![]()
(ii) What is the role of cryolite?
Answer:
Cryolite lowers the melting point of the mixture and increases the conductivity.
(b) Name the metal refined by Mond’s process.
Answer:
Nickel.
Question 20.
In the manufacture of ammonia by Haber’s process. Write the flow chart and chemical equations with optimum conditions.
Answer:
Ammonia is manufactured by the direct combination of nitrogen and hydrogen in the ratio 1:3 by volume.
Reaction : N2 + 3H2(g) ⇌ 2NH3(g) ;
ΔH = -46 kJ/mol
According to Le-Chatelier’s principle, high yield of Ammonia is obtained under the following favourable conditions.
(a) High pressure of 200atm
(b) Temperature of ~ 700K
(c) Use of Iron oxide as Catalyst in presence of small amounts of K2O and Al2O3 as promoters.
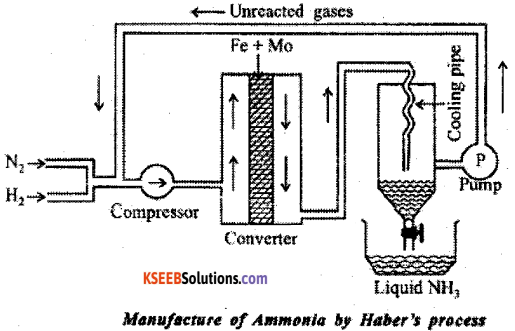
Procedure;
Step – 1 : Dry N2 and H2 in the ratio 1 : 3 compressed at 200 atm in compresser.
Step – 2 : Compressed gas passed through catalytical chamber, in presence of Fe, catalytical promoter Mo and 700 K forms ammonia.
Step – 3 : The ammonia formed is cooled in coolent below 239 K.
Step – 4 : The unreacted nitrogen and hydrogen are recirculated to compressor using pump. The above step repeated to get maximum yield.
![]()
Question 21.
(a) Give reason:
(i) Hydrogen bonding in H2O but not in H2S.
(ii) Cone. H2SO4 is a good dehydrating agent.
Answer:
(i) Oxygen in H2O is more electronegative than in H2S
(ii) Because cone. H2SO4 removes water molecule from others substances
(b) Give the structure of sulphurous acid (H2SO3)
Answer:
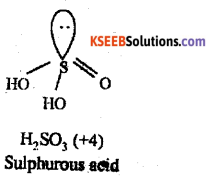
Question 22.
Complete the following chemical equations:
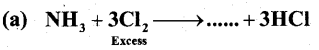
Answer:
(a) NCl3
(b) Na2SO3 + 2HCl → 2NaCl +H2O + …
Ans:
(b) SO2
(c) Br2 + 3F2 → ….
Answer:
(c) 2BrF3
Question 23.
Write the balanced chemical equation involved in the manufacture of potassium- dichromate from chromite ore.
Answer:
4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
2Na2CrO4 + 2H+ → Na2Cr2O7 + H2O + 2Na+
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
![]()
Question 24.
(i) What are interstial compounds?
Answer:
Interstitial compounds are those which are formed when small atoms like H, C (Or) N are traped inside the crystal.
(ii) Transition metals show good catalytic property. Give any two reasons.
Answer:
a. Presence of vacant – d – Orbitals
b. Tendency to exhibit variable oxidation state.
Question 25.
(a) Write the IUPAC name of K3[Cr(C2O4)3]
Answer:
Potassium trioxalatochromate (III)
(b) Give the facial (fac) and meridional (mer) isomeric structures of [Co(NH3)3(NO2)3].
Answer:
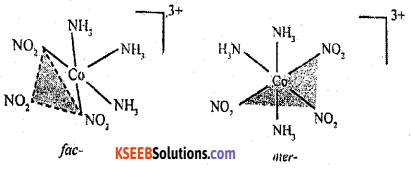
Question 26.
With the help of Valence Bond Theory (VBT) explain hybridisation, geometry and magnetic property of [Ni(CN)4]2- tetracyanido nickelate (II). ion. [Given : Atomic number of Ni = 28]
Answer:
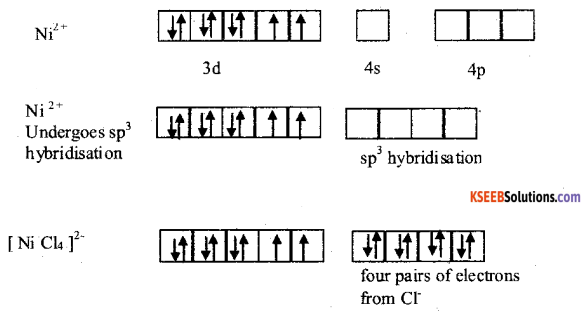
Geometry of [Ni Cl4]2-
Magnetic property: paramagnetic
![]()
Part-D
IV. Answer any three of the following: (Each question carries five marks) (3 × 5 = 15)
Question 27.
(a) Calculate the packing efficiency in Face Centred Cubic (FCC) lattice.
Answer:
The number of atoms per unit cell in fCC structures is ‘4’
Each atom is considered as one sphere.
So the volume of 4 atoms
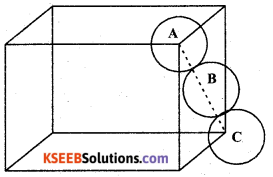
(4 sphere) = 4 × \(\frac { 3 }{ 4 }\) πr3 = \(\frac { 16 }{ 3 }\) πr3
Edge length a = 2\(\sqrt{2} r\)
The volume of the cubic unit cell = a3 = \((2 \sqrt{2} r)^{3}\)
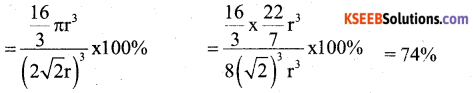
b) If a metal with atomic mass 209 crystallizes in a simple cubic lattice what is the edge length of its unit cell. (Given d = 91.5 kg m-3). 2
Answer:
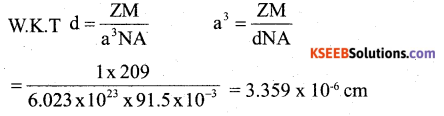
(c) What is Frenkel defect? What is its effect on the density of a solid?
Answer:
The dislocation of cation (small size) from its normal site to an interstitial site is called Frenkel defect. During Frenkel defect the density of the crystal does not change.
Question 28.
(a) 31 g of an unknown molecular material is dissolved in 500 g of water. The resulting solution freezes at 27.14 K. Calculate the molar mass of the material. [Given : Kf for water = 1.86 KKgmo-1, Tf0 of water = 273K].
Answer:

(b) What is reverse osmosis? Mention its use.
Answer:
The process of reversing the direction of osmosis by applying the pressure higher than the osmotic pressure to the solution of higher concentration is called reverse osmosis. During reverse osmosis, the pure solvent flows out of the solution through the semi permeable membrane. Reverse osmosis is used in desalination of sea water. When pressure, more than osmotic pressure (30 atm at 25 °C) is applied to the sea water, pure water is squeezed out of the sea water through the semi permeable membrane as shown in figure.
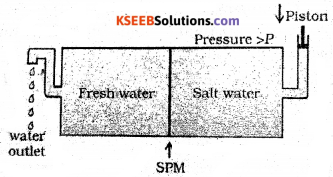
Question 29.
(a) Write the equations for the reactions taking place at anode and cathode in the lead-storage battery.
Answer:
Anode:
Pb(s) + SO42-(aq) → PbSO4 (s) + 2e–
Cathode :
PbO2 (s) + SO42- (aq) + 4H+ (aq) + 2e– → PbSO4 (s) + 2H4O(l)
(b) Calculate the value of ΔrG0 at 298 K for the cell reaction.
3Mg(s) + 2Al3+aq → 3Mg2+aq + 2Al(s)
[Given ; EoMg = -2.36 V, EEoAl =-1.66 V and F = 96487 C]
Answer:
Eocell = EoAl – EoMg = -1.66 – (-2.36) = 0.7v
ΔGo = -nEoF
= -6 × 0.7 × 96487 = 405.24KJ
![]()
Question 30.
(a) Derive an integrated rate equation for the rate constant of a first-order reaction.
Answer:
consider a first order reaction
R → p
A/c to Rate law, Rate = k[R]1

where I is the integration constant
when t = 0, [R] = [R]0
In [R]0 = -k × o + I
I = In [R]0
where [R]0 is the initial con of the reactant ‘R’ substituting the value of I in equation (1)
we get In [R]0 = – kt + [R]0
kt = In [R]0 – In [R]
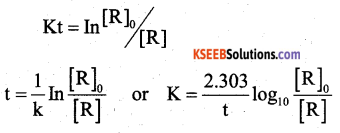
(b) The specific reaction rate of a reaction quadruples when temperature changes from 30°C to 50°C. Calculate the energy of activation of the reaction. [Given : R = 8.314 JK ‘mol1].
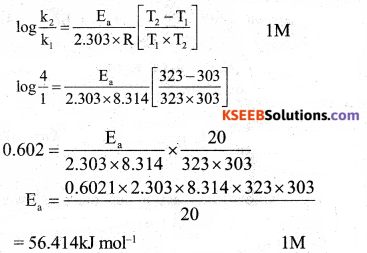
![]()
Question 31.
(a) Define shape selective catalysis. Name the Zeolite catalyst used to convert alcohols to gasoline in petroleum industry.
Answer:
The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis. HZSM – 5 is the Zeolite Catalyst used to convert alcohols to gasoline in petroleum industry.
(b) What is peptisation? Give an example.
Answer:
The process of conversion of freshly prepared precipitate into a colloidal solution by adding an electrolyte containing the common ion is called Peptisation. Ex : Ferric hydroxide sol.
(c) Write the expression for Freundich adsorption isotherm.
Answer:
Freudich adsorption isotherm is given by
![]()
V. Answer any four of the following. Each question carriers five marks: (4 × 5 = 20)
Question 32.
(a) Write the equations for the steps involved in the SN1 mechanism of hydrolysis of 2-bromo 2-methyl propane.
Answer:
(CH3)3CBr+OH → (CH3)3COH+Bre
2-bromo-2-methylpropane 2-methylpropan-2-ol
Step(l) : formation of carbocation
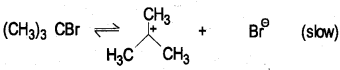
Step (2) : attack of nuclephile on carbocation.
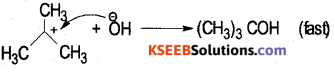
Rate of the reaction depends on slow step of the reaction r α [(CH3)3C Br]1 Order = 1
(b) (i) Name the product formed for the reaction of isopropyl iodide on alcoholic KOH.
(ii) What is the condition to be satisfied for a compound to be chiral?
Answer:
(i) Propene
(ii) The absence of the plane of symmetry.
(c) What is racemic mixtures?
Answer:
Equimolar mixture of dextrotatory and leavorotatory isomers is called recemic mixture.
![]()
Question 33.
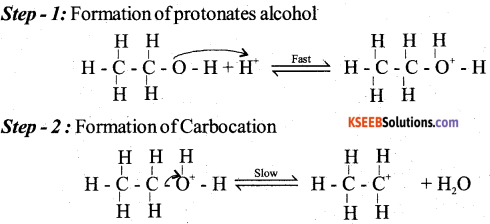
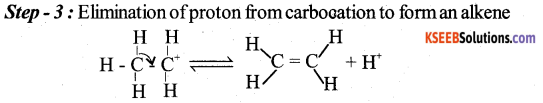
(a) Explain the mechanism of acid catalysed dehydration of ethanol to ethene.
Answer:
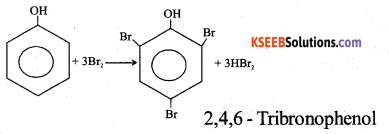
b) An organic compound with molecular formula C6H6O gives white precipitate with bromine water. Identify the functional group in the organic compound and write the chemical equation for the reaction.
Answer:
(c) How do you prepare methoxy ethane by Williamoson’s ether synthesis?
Answer:
CH3Cl + NaOC2H5 → CH3OC2H5 + NaCl
Methylchloride Sodiumethoxide Methoxyethane
Question 34.
(a) How does benzene reacts with acetyl-chloride in the presence of anhydrous AlCl3? Give equation.
Answer:
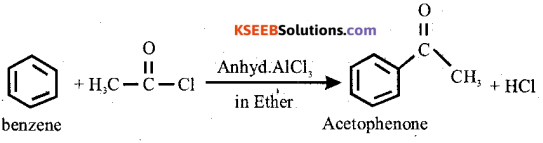
(b) (i)Write general equation for esterification reaction.
Answer:
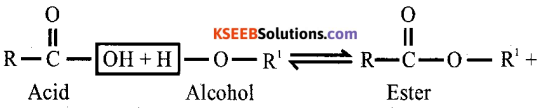
(ii) Name the product obtained when benzoic acid is heated with ammonia.
Answer:
Benzamide.
(c) Name the reagent used in the Clemmensen reduction.
Answer:
Zinc amalgam and concentrated hydrochloric acid.
![]()
Question 35.
(a)Between CH3NH2 and C6H5NH2 which is more base? Give reason.
Answer:
CH3 – NH2
Becuase methyl group is electron donating group which increases the electron density on nitrogen atom by +1 effect. This makes unshared electron pair easily available for protonation.
(b)
(i) Name the main product when aniline is heated with alcoholic KOH and chloroform.
(ii) Give the IUPAC name of (CH3)2 N – C2H5.
Answer:
(i) Phenyl isocyanide.
(ii) N, N-Dimethylethanamine.
(c) Complete the chemical equation.
![]()
Answer:
![]()
Question 36.
(a)Write the Haworth structure of maltose.
Answer:
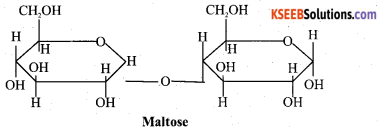
(b) What are hormones? Give one biological function of insulin. 2
Answer:
Hormones are the biochemical messengers secreted by endocrine (ductless) glands.
Insulin regulates the blood sugar leveL
(c) What are nucleosides? 1
Answer:
The compounds containing pentose sugar and nitrogen base are called nucleosides.
(d) What is peptide Linkages? How many peptide bonds are present in a tetra-peptide?
Answer:
The – CO NH – bond formed between two a – amino acids is called peptide bond or peptide linkage. Peptide bond is formed by the union of a – amino acids with the loss of water molecules. Three peptide bonds are present in a tetra – peptide.
(e) Name the hormone which regulates blood sugar level in the body.
Answer:
Insulin.
![]()
Question 37.
(a)How is Buna-N prepared? Give equation
Answer:
Buna-N manufactured by the co-polymerisation of 1, 3-butadiene and acrylonitrile in presence of peroxide or sodium catalyst.
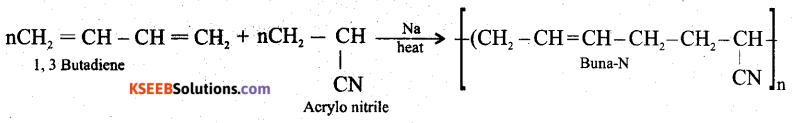
In Buna-N, Bu stands for butadiene and na = Na – sodium catalyst, N = nitrile (CN).
(b) Name the monomers of Nylon-6, 6.
Answer:
Tetra methyline dicarboxylic acid (Adipic acid) and hexa methylene diamine.
(c) What are thermosetting polymers?
Answer:
These polymers are cross linked molecules which undergo permanent change on heating. They become hard and infusible on heating and cannot be softed again.