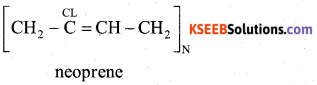Students can Download 2nd PUC Chemistry Model Question Paper 4 with Answers, Karnataka 2nd PUC Chemistry Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka 2nd PUC Chemistry Model Question Paper 4 with Answers
Time: 3 Hrs 15 Min
Max. Marks: 70
Part-A
I. Answer all the question each question carries one mark (10 × 1 = 10)
Question 1.
Name the law behind the dissolution of CO2 gas in solf under high pressure?
Answer:
Henry’s law.
Question 2.
Ornamental gold containig copper is an example for what type of solution ?
Answer:
Solid – solid solution.
Question 3.
Which gas is evolved at cathode during the electrolysis of an aqueous solution of Na Cl?
Answer:
Chlorine.
Question 4.
What happens to the half life period of a first order reaction if the initial concentration of the reactants is increased ?
Answer:
Half-life of a first order reaction is independent of the initial concern trution it remains constant.
![]()
Question 5.
Out of physisorption and chemisorption which one has lower enthalpy of edsorption ?
Answer:
Physisorption las lower enthalapy.
Question 6.
Give the composition of copper matte.
Answer:
CU2S – Fes
Question 7.
Noble gases are chemically inert give one reason ?
Answer:
The orbitals and of noble gases are completely filled they generally have 8 valence electrons and have stable electronic configuration hence they are chemically inert
Question 8.
What is “Chirality”?
Answer:
Chirality is a geometric property of some molecules andions a chiral molecule or ion is non super posable on its mirror image the presence of asymmetric carbon atom is one of several structural featares that induce chirality in organic an dinorganic molecules
Question 9.
Complete the following chemical reaction.
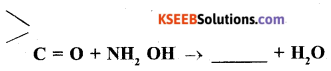
Answer:
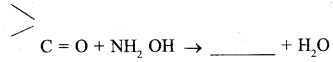
Question 10.
Which hormone regulates the sugar level in the blood?
Answer:
Insulin or glucagon.
Part – B
II. Answer any five of the following questions (5 × 2 = 10)
Question 11.
Calculate the no of particles ( atoms) per unit cell in a FCC crystal lattice?
Answer:
No.of atoms per unit cell of FCC = 8 comer atoms × \(\frac { 1 }{ 8 }\) atom +6 facecentred atoms × \(\frac { 1 }{ 2 }\)
i. e.8 × \(\frac { 1 }{ 8 }\)+ 6 × \(\frac { 1 }{ 2 }\) =1 + 3 = 4
Question 12.
What are ferromagnetic substances? give one example.?
Answer:
The substances which are strongly affected by magnetic field are called ferromagnetic substances and the phenomenon is known as ferromagnetism.
Ex: Iron or Nickel, or Cobalt or CrO2 any other.
![]()
Question 13.
The rate contant of a certain first order reaction is 200 S-1. What is its half life period ?
Answer:
for a first order reaction

Question 14.
Zr and Hf have almost identical radii give reason ?
Answer:
It is due to Lanthanoid contraction.
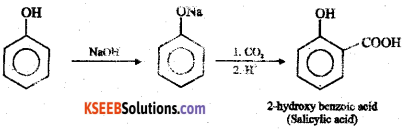
Question 15.
Explain Kolbe’s reaction ?
Answer:
Sodium phenate is treated with carbon dioxide and the product on acidification forms salicylic acid. This reaction is called Kolbe’s reaction.
ethanal ( acetaldehyde )? name the reaction?
Answer:
Aldehydes and ketones having at least often hydrogen under go a reaction in the presence of dilute alkali as catalyst to form β- hydroxy aldehydes (aldol) or β -hydroxy ketones (ketol), respectively. This is known as Aldol reaction.
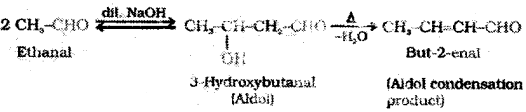
Question 17.
What is the role of following chemicals in food ?
i) Saccharin
Answer:
Saccharine is the well known sweetening agent which is more than 550 times sweet as compared to sucrose (or sugar). It is commonly used in the preparation of sweets for diabetic patients. Actually, it is not a carbohydrate. Now better sweetening agents are also available.
ii) Sodium benzoate
Answer:
Sodium benzate is a chemical used as a food preservative to stop spoilage of food.
Question 18.
What are antifertility drugs? give an example ?
Answer:
Anti fertility drugs are those enemical compounds which prevent pregnancy in women. Eg: novethin drone
![]()
Part-C
III. Answer any five of the following questions: each question carries three marks (5 x 3 = 15)
Question 19.
In the extraction of aluminium by electrolysis? (3)
i) Give the composition of electrolyte used
ii) Overall cell reaction
iii) Role’ of cryolite
Answer:
Removes silica impurity as slg calcium silicate.
Cryolite increases conductivity and reduces melting point of Al2O3.
Aluminium Extraction
Cathode : Al3+(melt) + 3e– + al(l)
Anode : C(s) + O2-(melt) CO(g) + 2e
C(s) + 2O2-(melt) CO2(g) + 2e
Overall reaction: 2Al2O3 + 3C 4Al + 3CO2
Question 20.
Write the balanced chemical equation with condition involved in manufacture of nitric acid by ostwald’s process. (3)
Answer:
Nitric acid in Ostwald process manufactured by the oxidation of ammonia.

2NO(g) + O2(g) → 2NO2(g)
Nitrogen dioxide so formed, dissolves in water to give HNO3.
3NO2(g)+H2O (I) → 2HNO3(aq) + NO(g)
Dilute nitric acid on distillation followed by dehydration using conc. sulphuric acid gives 98% nitric acid.
Question 21.
Complete the following chemical equations
i) pbs + 4O3 → pbSO4 + _____
ii) Cu + 2H2SO4 → CuSO4 + _____ + 2H2O
iii) Cl2 + 2H2O + SO2 → _____ + 2Hcl
Answer:
pbs + 4O3 → pbSO4 + 4O2
Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
Cl2 + 2H2O + SO2 → H2SO4 + 2Hcl
Question 22.
a) How is chlorine prepared using KMnO4
Answer:
By the action of HCl on KMnO4.
2KMnO4+ 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2 .
b) Why is I2 less reactive than Icl ?
Answer:
Because interhalogen compounds are more reactive than halogens the bond between atoms in inter halogens (x – x1) is weaker than the bond in halogens (x – x) hence ICL is more reactive than I2
Question 23.
a) Calculate the spin only magnetic moment of Fe2+
Answer:
Fe2+ ion has electmic configuration (Ar)4S03d6
it has four supaired electrons ∴ n = 4
spin only magnetic moment μ = \(\sqrt{n(n+2)} BM\)
= \(\sqrt{4(4+2)} BM\) = \(\sqrt{24} \mathrm{BM}\) = 4.9BM
b) Why Sc3+ salts are colourless whereas Cr3+ salts are coloured
Answer:
The ions Sc3+ have completely empty d- orbitals i e no unpaired electrons are present {[ Ar] 3d0} thus their salts are colourless as d – d transitions are not possible
![]()
Question 24.
Write the balanced equations in the manufacture of K2Cr2O7 from cromite ore?
Answer:
Manufacture of K2Cr2O7 from chromite ore involves the following steps
Step -1: Conversion of chromite ore into sodium chromate:
Chromite ore is heated with Na2CO3 in the presence of excess of air to form sodium chromate
4FeOCr2O3 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
Step – 2 : Conversion of sodium chromate into sodium di chromate.
The yellow solution of sodium chromate is filtered and acidified with H2SO4 to give a mix-ture of Na2 Cr2O7 and sodium sulphate
2Na2CrO4 + H2SO4 → Na2 Cr2O7 + Na2SO4+H2O
The less soluble Na2SO4 is removed by filtration.
Step – 3 : Conversion of sodium dichromate into potassium dichromate: The solution of sodium dichromate is treated with Kcl to give potassium dichromate.
Na2Cr2O7 + 2Kcl → K2Cr2O7 + 2Nacl
Question 25.
Explain the hybridization, geometry and magnetic property of [CoF6]3- on the basis of valence bond theory [ Z of Go = 27]
Answer:
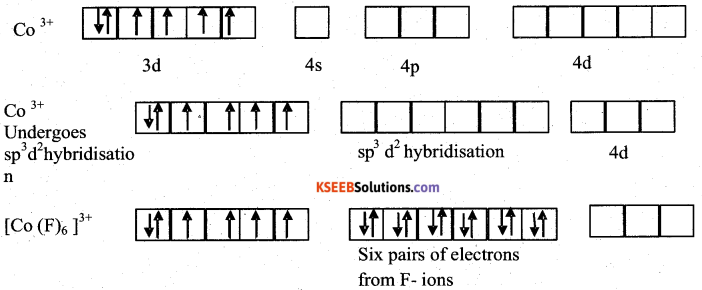
Geometry f[Co (NH3)6]3+
Magnetic property: paramagnetic
Question 26.
a) Write any two postulates of erner’s theory of Co- ordination compounds.
Answer:
Postulates :
1. Central metal ion in a complex shows two types of valences – primary valence and secondary valence.
2. The primary “alence is ionisable and satisfied by negative ions.
3. The secondary valence is non ionisable. It is equal to the coordination number of the central metal ion or atom. It is fixed for a metal. Secondary valences are satisfied by negative ions or neural molecules (ligands).
4. The primary valence is non directional. The secondary valence is directional. Ions or molecules attached to satisfy secondary valences have characteristic spatial arrangements. Secondary valence decides geometry of the complex compound,
b) Write the IUPAC name of [pt(NH3)3(H2O)Cl2]
Answer:
Triamine dicloro platinum hydrate.
Part-D
IV. Answer any three of the following each question carries five marks (3 × 5 = 15)
Question 27.
a) Calculate the packing efficiency in a body centered cubic (BCC) lattice.
Answer:
In a BCC unit cell, the edge length is ‘a’ and radius of the particle is ‘r’.
Then r = \(\frac{\sqrt{3}}{4} \mathrm{a}\) ∴ a = \(\frac{41}{\sqrt{3}}\)
and two particles are present in an unit cell of BCC
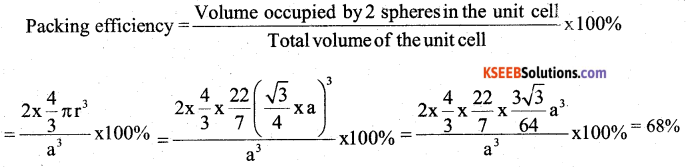
b) Silver forms a ccp lattice the edge length of his unit cell is 408.6 pm calculate the density of silver (NA = 6.022 × 1023 )
Answer:
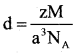
d = 4 × 107.9/(4.08)3 × 10-24 × 6.022 × 1023
d = 431.6/40.899
d= 10.5528g/cm3
![]()
Question 28.
a)5.8g of a non volatile solute was dissolved in 100 g carbon disulphide ( CS2 ) The vapour pressure of the solution was found to be 190mm of Hg calculate the molar mass of the solute given the vapour pressure of pure CS2 is 195 mm of Hg [ Molar mass of CS2 = 76gmol-1] (3)
b) Mention any two differences between Ideal and non ideal solutions (2)
Answer:
Ideal solution
- The inter moleculor interactions between the components of the solution are similar to those found in the pure components
- Ther is no volume change during mixing ((Av = o)
- Obeys raoult’s law of solution
Non ideal solution
- The inter molecular interaction between the components of the solution are different from those found in the pure components
- Volume of the solution is either less or more than the total volume of the components mixed
- Does not obey raoult’s law of solution either positive or negative devistion
Question 29.
a)State faradays first law of electrolysis write its mathematical from using usual notations. (2)
Answer:
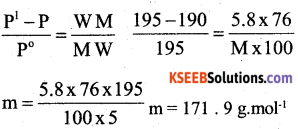
b) State Kohlrausch law (2)
Answer:
The limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.
c)
Write the overall cell reaction taking place in Daniel cell (1)
Answer:
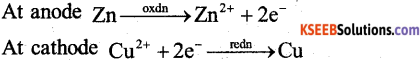
Zn(s) +Cu2+(aq) → Zn2+(aq) + Cu(s)
Question 30.
a) Derive the integrated rate equation for the rate constant of a Zero order reaction. (3)
Answer:
Consider a zero order reaction
R → P

[R] = -Kt + I …………. (1)
Where I is the integration constant
when t = o then [R] = [R]
∴ [R]0 = -K × 0 + I
∴ I = [R]0
Substituting the value of I in eq (1)
R] = -Kt + [R]0
Kt= [R]0 – [R]
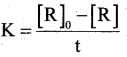
![]()
b. Draw a graph of potential energy v/s reaction coordinate showing the effect of a catalyst on activation energy.
Answer:
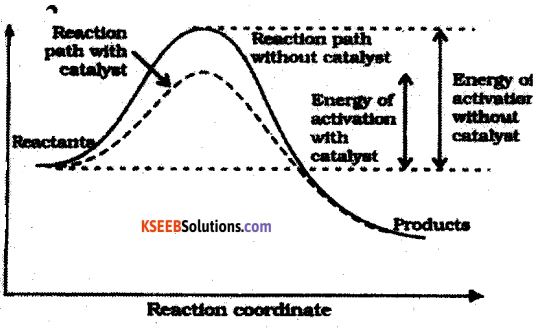
Question 31.
a) Mention any three differences between lyophilic and lyophobic colloids (3)
Answer:
Property
- Affinity towards dispersion medium.
- Method of preparation.
- Stability
Lyophilic
- 1. High affinity.
- Easily formed on mixing or heating the dispersed phase with dispersion medium.
- stable.
Lyophobic
- Low affinity
- Special methods are used.
- Highly unstable.
b) What is heterogeneous catalysis ? give an example ? (2)
Answer:
A catalytic process in which reactants and catalyst are in different phases are known as heterogenous catalysis.
E.g: 1.
![]()
Here the reactants are gases, catalyst iron is a solid
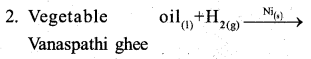
Here reactants and catalyst are in different phases.
Part-D
V. Answer any FOUR of the following. (Each question carries 5 marks) (4 x 5 = 20)
Question 32.
a) Explain the mechanism of SN 1 reaction taking 2- bromo – 2 methyl propane (t – butyl bromide) (2)
Answer:
Rate of the reaction depends on slow step of the reaction r α [(CH3)3C Br]1
Order =1
b) Explain Wurtz – Fittig’s reaction. (2)
Answer:
A mixture of an alkyl halide and aryl halide gives an alkylarene when treated with sodium in dry ether and is called as wurtz – fitting reaction.
c) Write the general formula of grignard reagent (1)
Answer:
R-Mg- X, where ‘R’ is alkgl or aryl group and ‘X’ is hologen atom
![]()
Question 33.
a) How is phenol manufactured by cumene process ? (3)
Answer:
Cumene is oxidised by oxygen to form cumene hydroperoxide. Which on acid hydrolysis gives phenol
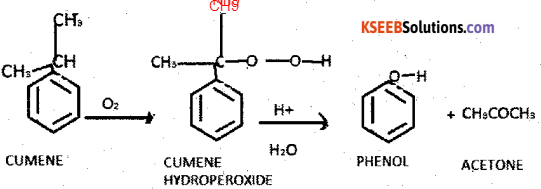
b) Among alcohols and phenols which one is more acidic ? and why ? (2)
Answer:
In phenol charge because of resonance this facilitates the heterolytic eleavage of – OH bond resulting in the* formation of phenoxide ion the benzene ring with draws the negative charge from oxygen atom hence phenutide ion formed is resonance stabilizes dne to this phenols are acidic in nature.
But in alchohols neither does oxygen of- OH group acquire positive change nor is the alkoxide ion is resonance stabilized in addition to this oxygen in alchols is attached to elestron donating alkyl groups these electron donating groups decrease the stability of alkoxide ion hence alchok are not acidic
Question 34.
a) Explain the mechanism of addition of HCN of a carbonyl in presence of a base. (3)
Answer:
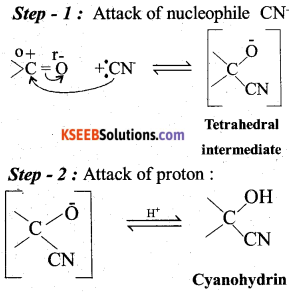
b) How is benzamide obtained from benzoic acid ? (2)
Answer:
Benzamide from benzoic acid.
![]()
Question 35.
a) Explain carbyl amine reaction
Answer:
carbyl amine reaction when primary amine is heated with a mixture of chlorofrom and alcholic solution of potasium hydroxide, chloroform and isocyanide having an into lerable smell is formed.
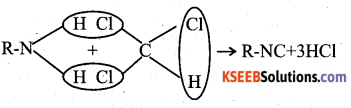
3HCL + 3KOH → 3KCL + 3H2O
R – NH2 + CHCL3 → R – NG + 3KCL + 3H2O
b) What is the action of bromine water on benzenamine (Aniline) at room temp
Answer:
Action of bromine water on benzenamine (Aniline) at room temperature 2,4,6 – tribromoanile.
c) The pkb valuse of Ammonia methanamine and bezenamine (aniline) are 4.75, 3.38 & 9.38 respectively arrange them in the increasing order of their basic strength
Answer:
Methanamine > benzenamine > ammoxia
Question 36.
a) How do you show that glucose contains a linear chain of six carbon atoms. (2)
b) What are essential amino acids ? is glyeine an essential amino acid ? (2)
Answer:
Based of dietary requirement they are classified into essential and Non – essential amino acids :
Essential amino acids : Amino acids that cannot be synthesised in the body, and must be obtained through diet. Example – Valine, leucine, isoleucine.
Non – essential amino acids: Amino acids that can be synthesised in the body.
Example – Glycine, alanine, gutamic acid Non – essential amino acids.
c) Write the general formula of Zwitter ionic form of an amino acid
Answer:
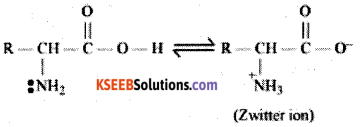
![]()
Question 37.
a) Explain addition polymerization with an example.
Answer:
A polymer formed by the addition of repeating monomer units possessing double or triple bond without elimination of by product molecule during polymerization is called addition polymer.
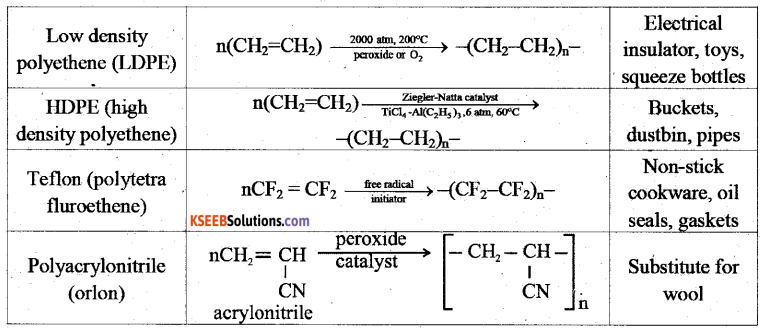
b) Name the monomers used in the manufacture of Nylon- 6, 6.
Answer:
Monomer of Nylon 6, 6 – Hexamethylene diamine and Adipic – acid.
Partial structure – [-OC-(CH2)4-CONH-(CH2)6 – NH-]n
c) Write the partial structure of Neoprene
Answer: