You can Download Chapter 8 Redox Reactions Questions and Answers, Notes, 1st PUC Chemistry Question Bank with Answers Karnataka State Board Solutions help you to revise complete Syllabus and score more marks in your examinations.
Karnataka 1st PUC Chemistry Question Bank Chapter 8 Redox Reactions
1st PUC Chemistry Redox Reactions One Mark Questions and Answers
Question 1.
Define oxidation in terms of electronic concept.
Answer:
Oxidation is a process in which loss of electrons take place.
Question 2.
Define by reduction in terms of electronic concept.
Answer:
Reduction is a process in which gain of electrons takes place.
Question 3.
What are redox reactions ? Give an example.
Answer:
Redox reaction is reaction in which oxidation and reduction take place
simultaneously, e.g. Zn(s)+ Cu2+ (aq) → Zn2+ (aq) + Cu(s):
Question 4.
What is oxidation number of alkali metals in their compounds?
Answer:
+1.
Question 5.
Zn(s)+Cu2+ → Zn2+ + Cu(s) is this reaction redox reaction ? If yes, name the
∴ oxidizing agent as well as reducing agent.
Answer:
Yes, Cu2+ is oxidising agent whereas Zn is reducing agent.
![]()
Question 6.
Which of the following does not conduct electric current and why?
Molten NaCl, Solid Pb, AgNO3 solution and methanol.
Answer:
Methanol will not conduct electric current because it does not ionise. .
Question 7.
Define cathode and anode.
Answer:
Cathode is electrode towards which cations are attracted. Anode is electrode which : attracts anions.
Question 8.
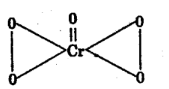
What is oxidation state of Cr in CrO5 and why?
Answer:
Cr has +6 oxidation state because it has 6 valence electrons, therefore can form 6 covalent bonds.
Question 9.
![]()
which is the strongest oxidising agent out of them ?
Answer:
Fe3+ is strongest oxidising agent because it has standard reducing potential. (i.e., +ve value) value.
![]()
Question 10.
What is meant by oxidation potential of an electrode ?
Answer:
Oxidation potential measures the tendency of an element or anion to lose the electrons.
Question 11.
What is relationship between standard oxidation potential and standard reduction potential?
Answer:
Both are equal in magnitude but opposite in sign.
Question 12.
Identify the oxidant and reductant in the following reactions :
(i) Zn(s) + \(\frac { 1 }{ 2 }\) O2(g) → ZnO(s)
(ii) Zn(s) + 2H+ (aq) → Zn2+(aq) + H2(g)
Answer:
(i) Zn is reducing agent (reductant) and O2 is oxidizing agent (oxidant)
(ii) Zn is reducing agent (reductant) whereas H+ is oxidizing agent (oxidant).
Question 13.
How will you define (i) Oxidant and (ii) Reductant in terms of oxidation no.?
Answer:
(i) Oxidant: Which decreases its own oxidation no. and increases the ox. no. of the other in a reaction.
(ii) Reductant : Which increases its own ox. no. and decreases that of other in a chemical reaction.
![]()
Question 14.
What do you mean by disproportionation reaction ?
Answer:
A disproportionation reaction is an oxidation-reduction process in which the same substance is oxidized and reduced e.g., 2Cu+ → Cu + Cu2+
Question 15.
Determine the oxidation number of C in the following : C2H6, C4H10, CO, CO2 and HCO3–
Answer:
O.N. of C = -3 in C2H6 ; -2.5 in C4H10 ; +2 in CO ; +4 in CO2 and +4 in HCO3–
Question 16.
Determine the oxidation number of O in the followiing : Na2O2 and CH3COOH.
Answer:
O.N. of+1 in Na2O2 and -2 in CH3COOH
Question 17.
Find out the oxidation number of sulphur in the following species : and HSO4–.
Answer:
O.N. of S = + 6 in HSO4–.
Question 18.
Determine the oxidation number of all the atoms in the following well known oxidants KCI04.
Answer:
K = +1, Cl = +7, O = -2
Question 19.
Determine the change in the oxidation number of S in H2S and SO2 in the following industrial reaction : 2H2S(g) + SO2(g) → 3S(g) + 2H2O(g)
Answer:
O.N. of S changes from -2 in H2S and +4 in SO2 to zero in elemental sulphur.
![]()
Question 20.
What is oxidised and what is reduced in the reaction ?
H2S + Cl2 → 2HCl + S
Answer:
H2S is oxidised to S and Cl2 is reduced to HCl.
Question 21.
In the reaction SnCl2 + 2HgCl2 → SnCl4 + Hg2Cl2
which is oxidant and which is reductant ?
Answer:
SnCl2 is a reductant and HgCl2 is an oxidant.
Question 22.
Identify the strongest and weakest reducing agents from the following list: Zn, Cu, Ag, Na, Sn.
Answer:
Strongest reducing agent in Zn and weakest reducing agent in Ag.
Question 23.
Can oxidation number of an atom in a chemical species he fractional ? Illustrate by an example.
Answer:
Yes, it can be fractional. For example oxidation no. of Pb in Pb3O4 = + \(\frac { 8 }{ 3 }\)
![]()
1st PUC Chemistry Redox Reactions Two Marks Questions and Answers
Question 1.
Calculate the oxidation number of underlined atoms in the following compounds and ions: CH4, Sb2O5, C6H12O6
Answer:
CH4
X + 4 = 0;
X = -4
Sb2O5
2x – 10= 0;
2x = 10
x = +5
C6H12O6
6x + 12 – 12 = 0
x = 0
Question 2.
Identify the oxidant and reductant in the following chemical reaction:
2I– (aq) + Cl2 (g) → 2Cl– (aq) + I2(s)
Answer:
2I– (aq) + Cl2 (g) → 2Cl– (aq) + I2(s)
Oxidant is Cl2 (g) reductant is I– (aq)
Question 3.
The standard reduction potentials of Al and Ni are -1.66 V and -0.28 V, respectively. Is Al a stronger or weaker reducing agent than Ni ? Explain.
Answer:
‘Al’ is stronger reducing agent than Ni because it has lower reduction potential. Al3+ is more stable than Ni2+ ion.
Question 4.
The standard reduction potentials of Zn2+, Mg2+ and Na+ are -0.76 V, -2.37 V and -2.71 Y respectively, which of the following is the strongest oxidizing agent?
Answer:
Zn2+ is strongest oxidising agent. It can gain electrons easily. It has highest standard reduction potential.
Question 5.
Which of the following is best reducing agent and why ? Li, Cu, Br2, F2, H2, K.
Answer:
Lithium (Li) is best reducing agent because it has lowest standard reduction potential, i.e., Li+ is most stable.
Question 6.
Calculate the oxidation number of (i) C in CH2Cl2 and (iii) Pb in Pb3O4.
Answer:
(i) C in CH2Cl2. Let the oxidation number of C in CH2Cl2 be x. Writing the oxidation number of each atom above its symbol.
![]()
x + 2(+1) + 2(-1) = 0 ,x = 0
![]()
Question 7.
Balance the equation by ion electron method.
Fe(OH)2(s) + H2O2 → Fe(OH)3 (s) + H2O is basic solution.
Answer:
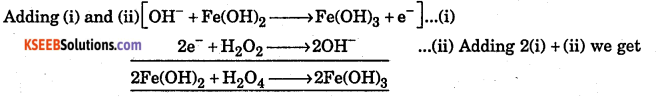
Question 8.
Balance the equation by ion electron method.
Al(s) + NO3 → Al(OH)4– + NH3 in basic solution.
Answer:
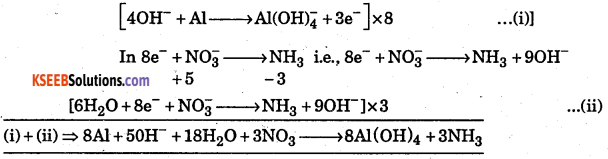
Question 9.
Balance the equation by ion electron method.
PbO2 + Cl– → ClO–+ [Pb(OH)3]– in basic solution.
Answer:
Here 2e– + PbO2 → [Pb(OH)3]–
2H2O + 2e–PbO22 → [Pb(OH)3] + OH– …(I)
2OH + Cl– → ClO– + 2e–+H2O …(ii) Add(i)&(ii)
PbO2 + OH– + Cl– + H2O → [Pb(OH)3 ]– + ClO–
Question 10.
Balance the equation by ion electron method.
CrO4– + H2O2 →CrO42- in basic solution.
Answer:
[2OH– + CrO–3 → CrO42- + e– + H2O] x 2 …(i)
2e–+ H2O2 → 2OH– …(ii) (Add(i) & (ii))
2OH–+2CrO3– +H2O2 → 2CrO42-+ 2H2O
Question 11.
Starting with the correctly balanced half reactions write the overall net ionic reactions. Chloride ion is form oxidized to Cl2 by MnO4– in acid
Answer:

Question 12.
Starting with the correctly balanced half reactions write the overall net ionic reactions for HNO2 reduces MnO4– in acid solution.
Answer:
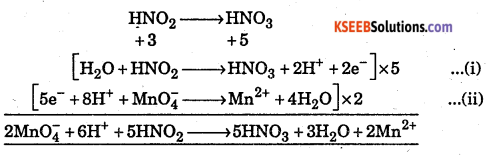
Question 13.
Starting with the correctly balanced half reactions write the overall net ionic reactions for HNO2 oxidizes I– to I2 in acid solution.
Answer:
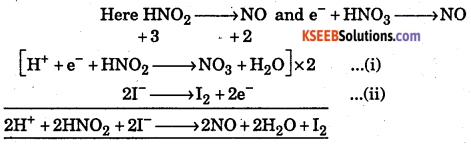
![]()
Question 14.
Starting with the correctly balanced half reactions write the overall net ionic reactions. ClO3– oxidizes Mn2+ to MnO2(s) in acid solution.
Answer:
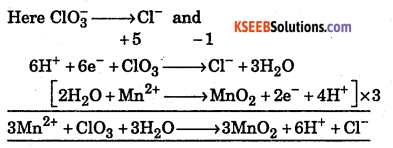
Question 15.
Consider the following cell notation :
Al(s)|Al3+(aq)|Ni2+(aq)|Ni(s), here
Which substances act as anode ? Which of them acts as cathode ? Write the net ionic equation for the cell reaction.
Answer:
At acts as anode, Ni acts as cathode.
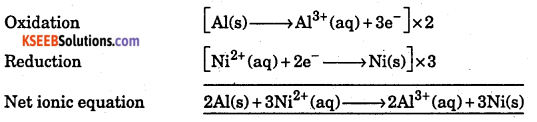
Question 16.
Describe the standard half cell that is used in electrochemistry to measure standard potentials. Write an equation for its half reaction and show its standard potential.
Answer:
Standard hydrogen elctrode is used to measure standard electrode potential.
It consists of H2(g) filled at 1 atm, 298 K in a sealed tube having Pt rod for metallic contact, coated with platinum black which acts as catalyst. It is dipped in 1 M solution of HCl. The reaction 2H+ (aq) + 2e– → H2(g) \(\mathrm{E}_{\mathrm{H}^{+} / \mathrm{H}_{2}}^{\circ}=0\)
Question 17.
Calculate the E°cell using these electrodes whose half reactions are:
Fe(OH)2(s) + 2e– → Fe(s) + 2OH–(aq) E° = -0.88V
NiO2(s) + 2H2O(l) + 2e– → Ni(OH)2(s) + 2OH–(aq) E° = +0.49V
Answer:

Question 18.
Calculate E°cell for the reaction Cl2(g) + 2l– → I2(s) + 2Cl–(aq) with the help of these half cell reactions:
Cl2 (g) + 2e– → 2Cl–(aq) ; E° = +1.36V
I2(g) + 2e– → 2I–(aq) ;E° = +0.54V
Answer:
![]()
Question 19.
(a) Give one use of heavy water in nuclear reactor.
(b) Write down balanced chemical equations of the reaction of cone. Nitric acid with (i) Copper and (ii) Iodine.
Answer:
(a) Heavy water is used as moderate and coolant in nuclear reactor.
(b) (i) Cu(s) + 4HNO3(conc.) → Cu(NO3)2 + 2NO2 + 2H2O
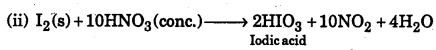
Question 20.
Balance the following ionic reaction with the help of oxidation number method : MnO4– + I– → MnO2 + IO3– (alkaline medium)
Answer:
MnO4– + I– → MnO2 + IO3– (alkaline medium)
H2O + 2MnO4– + I– → 2MnO2 + IO3– + 2OH– is balanced equation.
![]()
Question 21.
Complete the following equations :
(a) PbS(s) + H2O2(aq) → ………………………… (b) MnO4–(aq) + H2O2 →………………….
Answer:
PbS + 4H2O2 → PbSO4 +4H2O
2MnO4– + 3H2O2 → 2MnO2 + 3O2 + 2H2O + 2OH–
Question 22.
Write the cell reactions for Zn|Zn2+(lM)||cd2+(lM)|Cd
E°Zn2+ /Zn = -0.76V, E°Cd2+ /Cd = 0.40V
Answer:
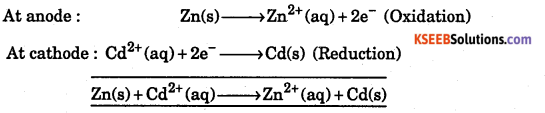
Question 23.
Zinc Silver oxide cell is used in heating aids or utensils and electric watches:
Zn → Zn2+ + 2e–; E° = +0.76V
Ag2O + H2O + 2e– → 2Ag + 2OH–; E° = +0.80V
Which is oxidized and reduced?
Answer:
Zn is oxidised, Ag2O is reduced.
Question 24.
The standard emf value of some elements are listed below at 298 K. Which of the following two electrodes should be combined to form a cell having highest emf ? Identify the cathode and anode and write the cell reaction. Also mention the direction of flow of electrons in the external and internal circuit.
![]()
Answer:
For maximum emf; anode should have minimum reduction potential and cathode should have maximum reduction potential. Hence A2+ / A couple should be anode and C2+/C couple acts as cathode.
Cell reaction A + C2+ → A2+ + C
Direction of flow of electrons is from A to C in external and from C to A internal circuit. Direction of flow of current is opposite to the direction of flow of electrons.
Question 25.
Silver jewellery turns greyish black due to the formation of Ag2S layer on it. Can this tarnish be removed by dipping tarnished jewllery in an aluminium vessel containing inert electrolytic solution. The standard electrode potential for the half reactions are given below :
Ag2S + 2e– → 2Ag + S2-; E° =-0.71V ;and Al3+ +3e– →Al; E° = -1.66V
Answer:
Tarnish (black colour) will be removed if the following reaction occur.
Al + Ag2S → A13+ +2Ag + S2 ; here E° for this reaction = -0.71-(-1.66) = 0.95 V
EMF is positive. ∆G will be negative this reaction will occur and tarnish will be removed.
![]()
Question 26.
Justify that the reaction : 2Na(s) + H2(g) → 2NaH is a redox reaction.
Answer:
In this reaction, Na is oxidised to Na+ and hydrogen is reduced from H2 and H- ion. Therefore it is a redox reaction.
2Na0 + H20 → 2Na+ H-1 (s)
Question 27.
Out of silver and aluminium vessel which one will be more suitable to store 1 M HCl solution and why ?
Answer:
Since reduction potential of silver is more than that of hydrogen (E°H+ /H2Pt = 0), silver vessel will be suitable to store 1 M HCl. On the other hand E° of Al3+ /Al is less than that of hydrogen (E°H+ /H2Pt) so that hydrogen will be liberated is stored in aluminium vessel.
Question 28.
What is the oxidation number of Cr in (i) K2CrO4 (ii) Ni and Ni(CO)4
Answer:
Let the Ox. no. of Cr in K2CrO4 be x.
2(+1) + x + 4(-2) = 0
+2 + X – 8 = 0
x = +6
Ox. no. of Cr in K2CrO4 = +6 A
Ox. state of Ni in Ni(CO)4 is x + 4(0) = 0;x – 0
Ox. state of Fe in Fe(CO)5 is x + 5(0) = 0 or x = 0
Question 29.
Can Fe3+ oxidize Br– to Br2 at 1 m concentrations ?
Answer:
E°(Fe3+/ Fe2+) is lower than that of E°(Br / B–). Therefore Fe2+ reduce Br2 but Br– cannot reduce Fe3+ . Thus Fe3+ cannot oxidize Br– to Br2.
Question 30.
What is the oxidation state of S in (i) H2SO3 (ii) P4 (ii) (iii) PH3 (iv) H3PO4
Answer:
(i) H2SO3 :2(+1) + x + 3(-2) = 0
x = +4
Ox. state of is IV in H2SO3
Answer:
(i) Ox. no. of P in P4 = 0
(iii) Ox. no. of P in H3PO4 .
3(+1) + x + 4(-2) = 0
+3 + x – 8 = 0
x = +5
Ox. no. of P in H3PO4 = +5
Question 31.
An iron rod is immersed in a solution containing NiSO4 and ZnSO4. When the concentration of each salt is 1M, predict giving reasons which of the following reactions is likely to proceed ? (i) Iron reduces Zn2+ ions, (ii) Iron reduces Ni2+ ions.
Answer:
Given : E°(Zn2+ /Zn) = 0.76V, E°(Fe2+ /Fe) = 0.44V and E°(Ni2+ /Ni) = 0.25V
Answer:
(i) The reduction potential of iron is more than that of zinc. Therefore, iron will be reduced. In other words Zn2+ will not be reduced.
(ii) The reduction potential of Ni2+ is more than that of Iron. Therefore, Ni2+ will be reduced by iron.
![]()
Question 32.
Which of the following redox reaction is oxidation and which is reduction?
(i) Zn → Zn2+ + 2e–
(ii) Cl2 + 2e– → 2Cl–
(iii) Fe → Fe2+ + 2e–
(iv) Sn4+ + 2e– → Sn2+
Answer:
(i) Oxidation
(ii) Reduction
(iii) Oxidation
(iv) Reduction
Question 33.
Arrange the molecules, NH3, NO3, HN3, NO2–, N2O4 and N2H4 in the decreasing order of the oxidation states of nitrogen.
Answer:
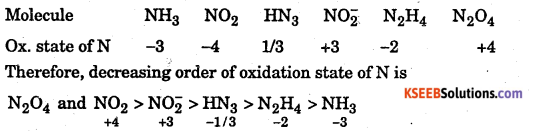
Question 34.
Determine the change in oxidation number of S in H2S and SO2 in the following industrial process: 2H2S + SO2 → 3S + 2H2O
Answer:
Let us write the oxidation numbers of atoms of all the reactants and products taking part in the reaction:

O.N. of S in H2S increases by 2 O.N. of S in SO2 decreases by 4
Question 35.
Can the reaction Cr3O72- + H2O ⇌ CrO42- +2H+ be regarded as a redox reaction ?
Answer:
Ox. no. of Cr in Cr2O72- ⇌ +6 Ox. no. of Cr in Cr2O72-= +6
Since the ox. no. of Cr has neither increased nor decreased in the above reaction, therefore this reaction cannot be regarded as a redox reaction.
Question 36.
Balance the oxidation reduction reaction FeS2 + O2 → Fe2O3 + SO2
Answer:
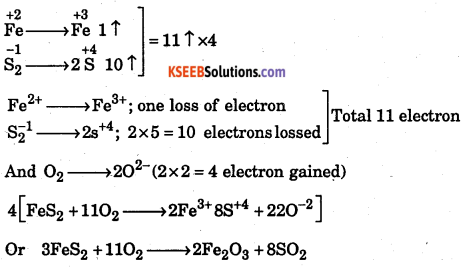
Question 37.
Which of the two ClO2– or ClO4– show disproportionation reaction and why ?
Answer:
The oxidation state of Cl in ClO2 is +3 and in ClO4 is +7. Chlorine is present in highest oxidation state of +7 in ClO4– and it cannot increase its oxidation state. Hence ClO4– is not disproportionate.
The disproportion reaction of ClO2– is
![]()
![]()
Question 38.
Which type of electrolytic are used in salt bridge ?
Answer:
Only those electrolytes for which cations and anions have nearly the same ionic mobilities (i.e. distance travelled by an ion per second under a potential gradient of one voltmeter) are used as electrolytes in the salt bridge. Thus KCl, KNO3, K4SO4, and NH4NO3 are preferred over NaCl, NaNO3 and Na2SO4.
Question 39.
Mention the cation and anion which have highest ionic mobility.
Answer:
Among cations, H+ ion has the highest ionic mobility and among anions, OH– has the highest ionic mobility.
Question 40.
The standard electrode potential corresponding to the reaction Au3+(aq) + 3e– → Au(s) is 1.5 V. Predict if gold can be dissolved in 1M HCl solution and on passing hydrogen gas through gold salt solution, metallic gold will be precipitated or not.
Answer:
Consider the half reaction, 2H+(aq) + 2e– → H2(g); E° = 0.0V
Au3+(aq) + 3e– → Au(s); E° = 1.50V
Since E° (1.50V) for (Au3+ / Au) is higher than that H+ / – \(\frac { 1 }{ 2 }\)H2 (0.0V), therefore, Au3+
can be more easily reduced than H+ ions Au3+ can be reduced to Au metal by H2 butr H+ cannot oxidize metallic gold to Au3+ ions. In other words, metallic gold does not dissolve in 1M HCl instead H2 gas can reduce gold salt to metallic gold.
Question 41.
What is the oxidation number of S in (i) Na2S4O6, (ii) S2Cl2?
Answer:
(i)
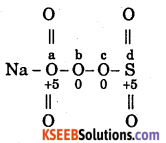
In b & c, s has oxidation number is zero. In a & d s has oxidation number +5
(ii)
![]()
Question 42.
What is the name of the reaction ?
2CH3CH2CH2SH → cCH3CH2CH2 – S – S – CH2CH2CH3
Whether condensation, oxidation, reduction or polymerization?
Answer:
This is an example of oxidation reaction since two H-atoms have been removed.
![]()
Question 43.
Both Cr2O72-(aq) and MnO4– (aq) can be used to titrate Fe2+(aq). If a given titration, 24.50 cm3 of 0.1 M Cr2O72- were used, then what volume of 0.1 M MnO4– solution would have been used for the same titration ?
Answer:
Suppose V2cm3 of M2Fe2+ is titrated against 24.50 cm3 0.1M Cr2O72- and V1cm3 of 0.1M MnO4– solution, then


1st PUC Chemistry Redox Reactions Three/Four Marks Questions and Answers
Question 1.
Balance MnO2 + HCl → MnCl2 + Cl2 + H2O
Answer:
Solution x
Step – 1: Assign oxidation number:
![]()
Step – 2 : Write the oxidation number changes.
Change in oxidation number of Mn + 4 to +2 = 2
Change in oxidation number of Cl – 1 to 0 = +1
Step – 3 : Cross multiply the numbers. Co-efficient 2 is multiplied to HCl and 1 is multiplied to MnO2.
MnO2 + 2HCl → MnCl2+H2O + Cl2
Step – 4 : Check the oxidation number, and 2 molecules of HC1 on left hand side to balance the chlorine atoms of MnCl2. In order to balance oxygen and hydrogen atom 2 molecule of H2O has to be added on the right hand side.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Question 2.
Balance Fe2++ Cr2O72- → Fe3+ + Cr3+ is acid media.
Answer:
Solution:
Step -1: (LEO = Loss of Electron Oxidation)
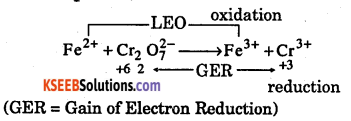
(GER = Gain of Electron Reduction)
Step-2: Fe2+ → Fe3+ … (1) (2 → 3 = 1 unit ON) ,
Cr2O72- → 2Cr3+ … (2) (+6 →+3 = 3 units 2 sets => 6 units ON)
Step – 3 : Cross multiplication i.e., (1) x 6 and (2) x 1
(1) x 6 ⇒ 6Fe2+ → 6Fe3+ … (3)
(2) x 1 ⇒ Cr2O7-2 → 2Cr3+ …(4)
Step – 4 : Add (3) + (4) ⇒ 6Fe2+ + Cr2O72- → 6Fe3+ + 2Cr3+
Step – 5 : Balance Oxygen and then Hydrogen
6Fe2+ + Cr2O72- + 14H+ → 6Fe3+ + 2Cr3++ 7H2O
![]()
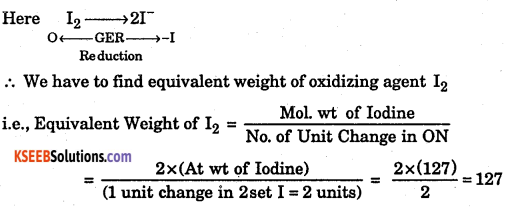
Question 3.
Evaluate the equivalent weight of I2 from the following equation.
I2 + 2S2O3 → S4O62-+2I–
Answer:
Question 4.
Find equivalent weight of KMnO4 from
![]()
Answer:
Balance the given equation
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
Method – 1: Stoichiometry
We know equivalent weight of Oxygen = 8
Atomic weight of Oxygen = 16
∴ 2 equivalent of oxygen = 1 Atomic weight of oxygen
Here 5[O] = 10 equivalent of oxygen
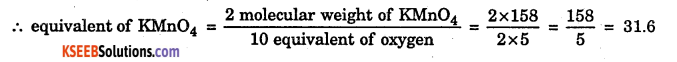
Method – 2 : (Oxidation Number)
2 KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
+7 ← 5 Unit GER Reduction → +3
KMnO4 acts as oxidising agent
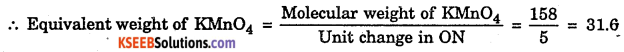
Question 5.
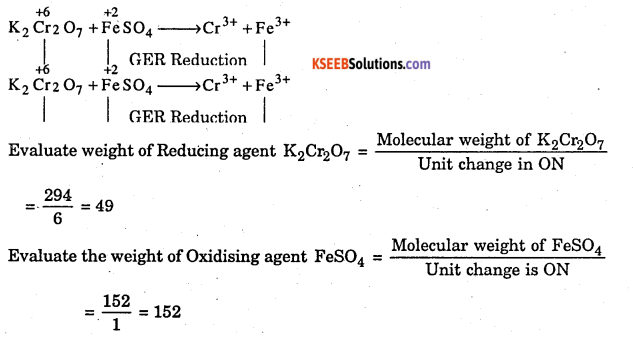
Evaluate the equivalent weight of oxidizing and reducing agents in the
following reaction (4M) K2Cr207 +FeS04 >Cr3+ +Fe3+
Answer:
Assigning oxidation number,
![]()
Question 6.
The half cell reaction with their oxidation potentials are
Pb(s) → Pb2+(aq) + 2e–; Eocell = +0.13V
Ag(s) → Ag(aq) + e–; Eocell = -0.80V
Write the cell reaction and calculate its EMF.
Answer:
Rewrite the two equations in the reduction form thus.
Pb2+ (aq) + 2eE– → Pb(s) ; E° = -0.13V .. (i)
Ag+(aq) + e– → Ag(s) ; E° = +0.80V …(ii)
To obtain the equation for the cell reaction, multiply Eq. (ii) with 2 and subtract Eq. (i) from it, we have,
Pb(s) + 2Ag+(aq) → Pb2+(aq) + 2Ag(s) ; Eocell =+0.80-(-0.13) =+0.93V
Question 7.
Justify that the reaction ; 2Cu2O(s) + Cu2S(s) → 6Cu(s) + SO2(g) is a redox
reaction. Identify the species oxidized, reduced, which acts as oxidation and which acts as reductant.
Answer:
Writing the oxidation number of each atom above its symbol we have,
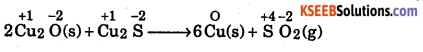
Here, in the reaction, the oxidation number of copper decreases from +1 in Cu2O or Cu2S to 0 in copper metal, therefore, copper is reduced and is .oxidizing agent or oxidants. Further, the oxidation number of S increases from -2 in Cu2S to +4 in SO2. Therefore, sulphur is oxidized. Thus, it is reducing agent of reductant.
Question 8.
Using stock notation, represent the following compounds : HAuCl2 , TI2O, FeO, Fe2O3, Cul, CuO, MnO and MnO2.
Answer:
By applying the various rules for determining the oxidation number of different atoms in a compound, the oxidation number of each metallic element in the given compounds is as follows:
In HAuCl2 , Au has +3; in TI20, TI has +1; in FeO, Fe has +2; in Fe2O2 , Fe has +3 ; . in Cul, Cu has +1 : in CuO, Cu has +2 ; in MnO, Mn has +2 while in Mn2O , Mn has +4 oxidation state. Therefore, these compounds may be represent as :
HAu(II)Cl4, Tl2(1)O, Fe(II)O, Fe2(III)O3, Cu(I)I, Cu(II)O, Mn(II)O, and Mn(IV)O2.
Question 9.
Balance the equation, Mg(aq) + HNO3(aq) → Mg(NO3)2(aq) + N2O(g) + H2O(1)
Answer:
Find out the elements which undergo a change in oxidation number (O.N.)
O.N. increases by 2 per Mg atom
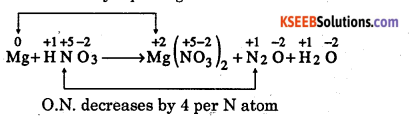
Here, O.N. of Mg increases from 0 in Mg metal to +2 in Mg(NO3)2 and that of N decreases from +5 in HNO3 to +1 in N2O.
Question 10.
Split the following redox reactions into the oxidation and reduction half reactions.
(a) 2K(s) + Cl2(g) →2KCl(s) (b) 2Al(s) + 3Cu2+(aq) → 2Al3++(aq) + 3Cu(s)
Answer:
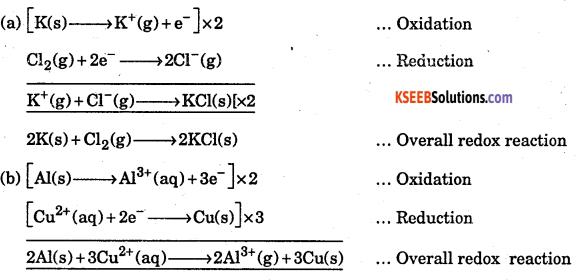
![]()
Question 11.
Predict the oxidation state of (a) C in C3O2, (b) Cr in Br3O3, (c) S in S3O32-
Answer:
(a) The structure of C3O2 (carbon -2 +2 0 +2 -2 suboxide) isO = C = C = C = 0 each of the two terminal oxygen atoms have an oxidation state of -2 and the carbons to which they are atteached have an oxidation state of +2. (Since when ever a covalent bond is formed between similar atoms, each of the atoms is given an oxidation state of zero), therefore, the oxidation state of central carbon atom in C3O2 is zero.
Thus, the two terminal carbon atoms are present in +2 oxidation state each, whereas the third one is present in zero oxidation state. Therefore, the average oxidation state of carbon in
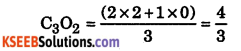
(b) Tribromooctaoxide (Br3O8)
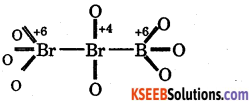
We can easily find out that the terminal bromine atoms are present in +6 oxidation state while the middle one has an oxidation state of +4. However, the average oxidation number of Br in Br308 turns out to be
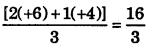
(c) Tetrathionate ion
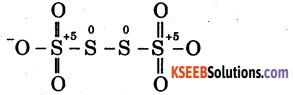
Since each of the two terminal sulphur atoms is connected to two oxygen atoms by a double bond and one oxygen atom by a single bond, therefore, the oxidation state of each of these terminal sulphur atoms is +5.
Since two central sulphur atoms are linked to each other by a single bond and each sulphur is further attached to similar species on either side, the electron pair forming the S – S bond remain in the centre and hence each of the two central sulphur atoms has an oxidation state of zero.
However, the average oxidation state of the sulphur atoms is
![]()
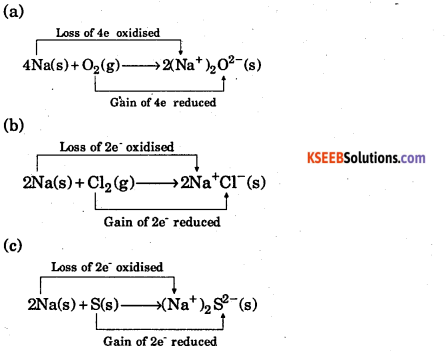
Question 12.
Mention which is reduced and oxidized in the following reaction :
(a) 4Na(s) + O2(g) → 2Na20(s)
(b) 2Na(s) + Cl2(g) → 2NaCl(s) ,
(c) 2Na(s) + S(s) → Na2S(s)
Answer:
Question 13.
Identify the oxidant and reductant in the following reactions :
(a) 10H+ (aq) + 4Zn(s) + NO3– (aq) → 4Zn2+(aq) + NH+4 +3H2O(l)
(b) I2(g) + H2S(g) → 2IH(g) + S(s)
Ans:
(a) Writing the O.N. of all the atoms above their symbols, we have
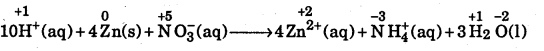
O.N. of Zn changes from zero in Zn to +2 in Zn2+ and, therefore, it is oxidized and hence Zn acts as a reductant.
The O.N. of N decreases from +5 in NO3– to -3 in NO4+ and, therefore, it is reduced and hence NO3– acts as the oxidant.
(b) Writing the ON of all the atoms above their symbols, we have
![]()
The ON of I2 decreases from zero in I2 to -1 in HI, therefore, I2 is reduced and hence it acts as on oxidant.
The O.N. of S increases from -2 in H2S to zero in S, therefore, H2S is oxidized and hence it acts as the reductant.
![]()
Question 14.
0.2g of a sample of H2O2 reduced 20 ml of 0.1M KMnO4 solution in acidic medium. What is the percentage purity of H2O2 ?
Answer:
No of moles of KMnO4 present in 20ml of 0.1M
KMnCh solution = \(\frac{20}{1000}\) x 0.1 = 2 x 10-3
The balanced equation for the redox reactions is :
2KMnO4 + 5H2O2 + 3H2SO4 → K2SO4 + 2MnSO4 + 8H2O + 5O2 …(1)
From the equation, 2 moles of KMnO4 = 5 moles of H2O2.
2 x 10-3 moles of KMnO4 will react with H2O2 = \(\frac{5}{2}\) x 2 x 10-3 = 5 x 10-3 moles
Molecular wt. of H2O2 = 34
Amount of H2O2 actually present = 34 x 5 x 10-3 = 0.17g .
Percentage purity of in 20 ml is = \(\frac{0.17}{0.20}\) x l00 = 85
Question 15.
16.6g of pure potassium iodide was dissolved in water and the solution was . made upto one litre. V cm3 of this solution was acidified with 20 cm3 of 2M HCl. The resulting solution required 10 cm3 of decinormal KlO3 for complete oxidation of I- ions to ICl. Find out the value of V.
Answer:
The chemical equation for the redox reactions is :
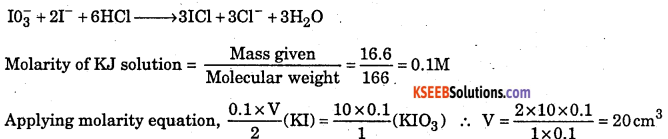
Question 16.
Which of the following species, do not show disproportionation reaction and why ? CIO–, CIO2–, CIO3– and CIO4– Also write reactions for each of the species that disproportionates.
Answer:
The oxidation state of Cl in all the given species are :
![]()
Out of above species, CIO4– does not undergo disproportionation since in this oxoanion chlorine is already present in the highest oxidation state of +7 and hence cannot be further oxidized.
All the remaining oxoanions have oxidation state lower than the highest (i.e., +7) and higher than the lowest (i.e. -1) therefore, all these species undergo disproportionation reactions as shown below.
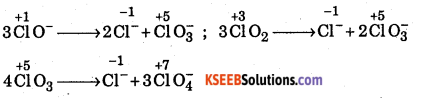
Question 17.
How does CU2O act as both oxidant and reductant ? Explain with proper reactions showing the change of oxidation numbers in each example.
Answer:
Cu2O undergoes disproportionation to form Cu2+ and Cu.
2Cu2+(aq) >Cu2+(aq) + Cu(s)
Thus, Cu2O acts both as an oxidant as well as a reductant.
(i)
![]()
and Cu2O acts as a reductant and reduces O2 to O2-
(ii) When heated with Cu2S it oxidizes S2- to SO2 and hence Cu2O acts as an oxidants

![]()
Question 18.
Determine the volume of \(\frac { M }{ 8 }\) KMnO4 solution required to react completely with 25.0cm3 \(\frac { M }{ 4 }M/4\) FeSO4 solution in acidic medium.
Answer:
The balanced ionic equation for the reaction is
MnO4–+ 5Fe2+ + 8H+ → Mn2+ + 5Fe3+ + 4H2O
From the balanced equation, it is evident that 1 mole of KMnO4 = 5 moles of FeSO4.
Applying molarity equation to the balanced redox equation,
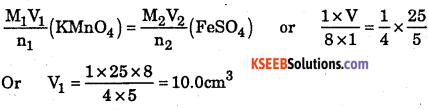
Thus, the volume of \(\frac { M }{ 4 }M/4\) KMnO4 solution required =10.0 ml.
Question 19.
Calculate the concentration of hypo (Na2S2O3.5H2O) solution in gdm-3 if 10.0ml of this solution decolourised 15mL of M/40 iodine solution.
Answer:
The balanced equation for the redox reaction is 2S2O32- + I2 → 2I– + S4O6 2-
From the balanced equation, it is evident that 2 moles of Na2S2C>O3 ≡ 1 mole of I2.
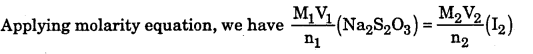
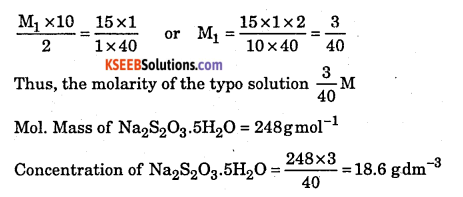
Question 20.
Calculate the volume of 0.05M KMnO4 solution required to oxidize completely 2.70g of oxalic acid (H2C2O4) in acidic medium.
Answer:
Balanced equation for the redox reaction is :
2KMnO4 + 5(COOH)2 + 3H2SO4 → K2SO4 + 2MnSO4 + 10CO2 + 8H2O
No of moles oxalic acid = 2.70/90 = 0.03 mole
From the balanced equation, 5 moles of (COOH)2 = 2 mole KMn04 .
Then 0.03 mole (COOH)2 = pppp— x 0.03 = 0.012 moles of KMnO4.
Now, 0.05 mole of KMnO4 is present in solution given = 1000 cm3.
0.012 mole of KMnO4 is present in solution = \(\frac { 2 }{ 5 }\) = 240 cm3
Question 21.
Write the rules for assigning oxidation number.
Answer:
- All atoms in the elemental or molecular state have ‘zero’ oxidation state.
- Elements of group I and II in the periodic table always have ‘+1’ and ‘+2’ oxidation sstates respectively.
- Hydrogen ‘+1’ oxidation state in all its compounds except metal hydrides where it is ‘-1’
- Oxygen has been assigned an oxidation number of ‘-2’ in all its compounds except peroxides and oxygen fluoride. In peroxide it is ‘-1’ and in oxygen fluoride it is ‘+2’.
- Halogens generally have ‘-1’ oxidation state. Except fluorine, other halogens may have positive oxidation states in their oxides and inter halogen compounds (Fluorine always has ‘-1’ state).
- The algebraic sum of oxidation numbers of all the elements in a compound is zero and that in an ion is equal to the net charge on the ion.
![]()
Question 22.
Balance MnO4– + Fe2+ → Fe3+ + Mn2+ in acidic medium by ion electron method.
Answer:
MnO4– + Fe2+ → Fe3+ +Mn2+
[Fe2+ → Fe3+] x 5 ……………(i)
8H+ + MnO4– >Mn2+ + 4H2O …(ii)
… (ii) Adding (i) and (ii), we get
5Fe2+ + 8H+ + MnO4– → Mn2+ + 4H2O + 5Fe3+
Question 23.
Find the oxidation state of sulphur in the following compounds :
Answer:
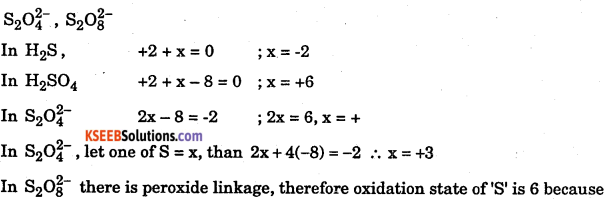
Question 24.
Find the oxidation number of element underlined in the following species :
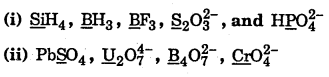
Answer:
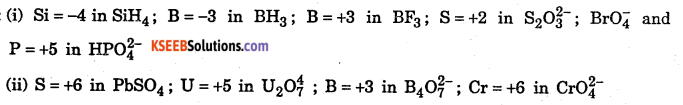
Question 25.
Calculate the oxidation number of (i) N in NO3– ; (ii) P in H3P2O7– CO32-.
Answer:
(i) N in NO3– , let the oxidation number of N in NO3– be x. Writing the oxidation number of each atom above its symbol. \(\begin{array}{cc}{\mathbf{x}} & {-2} \\ {\mathbf{N}} & {\mathbf{O}_{3}}\end{array}\)
x + 3(-2) = -1
x – 6 = -1, x = +5
(ii) P in H3P2O7– , let the oxidation number of P in H3P2O7– be x. Writing the oxidation
number of each atom above its symbol.
\(\begin{array}{ll}{\text { th } x} & {-2} \\ {\text { H }_{3} P_{2}} & {\text { o_{ } }}\end{array}\)
H3P2O7–= 3(+1) + 2 (x) = -1 +7(-2) or 2x – 11 = -1; x = + 5
Thus, the oxidation number of P in H3P2O7– is +5.
![]()
Question 26.
What is the oxidation number of metals (i) [Fe(CN)6 ]4- and (ii) MnO4– ?
Answer:
(i) Fe in [Fe(CN)6 ]4-. Let the oxidation number of Fe in [Fe(CN)6 ]4- bexand CN be -1 i.e..,
![]()
∴ Sum of oxidation numbers of all the atoms in
[Fe(CN)6 ]4- =x + 6(-l) = -4 = x-6 = -4; x = +2
Thus, the oxidation number of Fe in [Fe(CN)6 ]4- f is +2.
(ii) Mn in MnO4–. Let the oxidation number of Mn in MnO4– be x. Writing oxidation number of each atom above its symbol, we get.
![]()
Thus, the oxidation number of Mn in MnO4– is +7
Predict the oxidation number of the element underlined NaCl, MgSO4, AlF3, SiCl4, PF3, P4O10, SF6, SO3, HClO4, Cl2O7.
Answer:
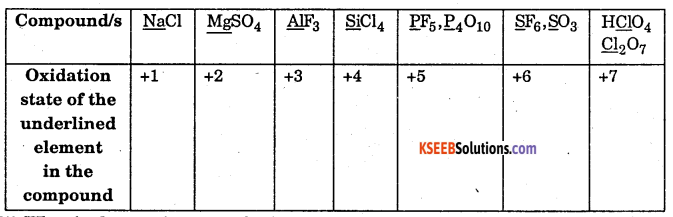
Question 27.
What is the maximum and minimum oxidation numbers of N, S and Cl?
Answer:
(i) The highest oxidation number (O.N) of N is +5 since it has five electrons in the valence shell (3s2 2p3 ) and its minimum O.N. is -3 since it can be accept three more electrons to acquire the nearest inert gas (Ne) configuration.
(ii) Similarly, the highest O.N. of S is +6 since it has six electrons in the valence shell (3s2 2p4) and its minimum O.N. is -2 since it needs two more electrons to acquire the nearest inert gas (Ar) configuration.
(iii) Likewise the maximum O.N. of Cl is +7 since it has seven electrons in the valance shell (3s2 2p5 ) and its minimum O.N. is -1 since it needs only one more electron to acquire the nearest (Ar) Noble gas configuration.
Question 28.
Nitric acid acts only as an oxidising agent while nitrous acid acts both as an oxidising as well as a reducing agent. Why ?
Ans:
(i) Nitric Acid HNO3 : Here Oxidation number of N is HNO3 = +5 ; But
Maximum oxidation number of N = +5 ; Minimum oxidation number of N = -3 Since the oxidation number of N in HNO3 is maximum (+5), therefore it can only decrease by accepting electrons. Hence HNO3 acts only as an oxidizing agent.
(ii) Nitrous Acid HNO2 : Here Oxidation number of N in HNO2 = +3
Maximum oxidation number of N = +5 ; Minimum oxidation number of N = -3 Thus, the oxidation number of N can either increase by losing electrons or can decrease by accepting electrons. Therefore, HNO2 acts both as an oxidizing as well as a reducing agent.
Question 29.
Write correctly the balanced equations for the following redox reactions using half reaction:
(i) H2S + Fe3+ → Fe2+ + S + H+
(ii) I–+IO3– + H+ → I2+H2O
(iii) I– + O2 + H2O → I2 + OH–
(iv) Cu(s) + Au+ → Au(s) + Cu2+
Answer:
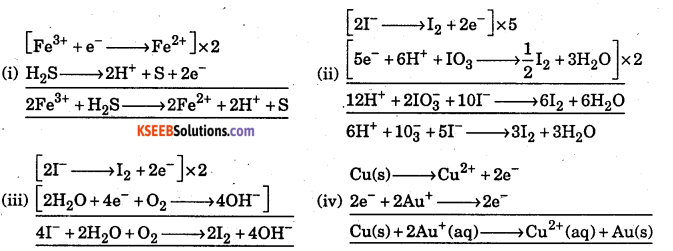
![]()
Question 30.
Consider the following reactions that produce electricity in the galvanic cell:
(i) 2Fe3+(aq) + 2Cl–(aq) → 2Fe2+ (aq) + Cl2(g)
(ii) 2Cr(s) + 3Cu2+ → 3Cu(s) + 2Cr3+
Write the anode and cathode reactions for the galvanic cell. Write cell in the usual notation.
Cathode
Answer:
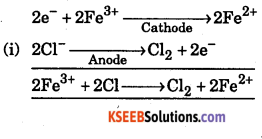
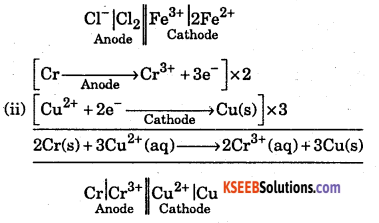
Question 31.
Balance the following reaction :
(i) Cu + NO3– → NO2 + Cu2+
Write correctly balanced half cell reactions and overall equations for the following equations by ion electron method:
Answer:
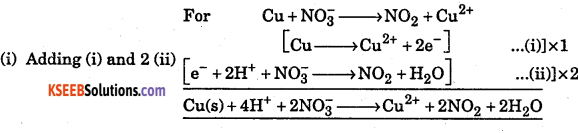
Question 32.
NO3– + Bi(s) → Bi3 + NO2 in acid solution by ion electron method.
Answer:
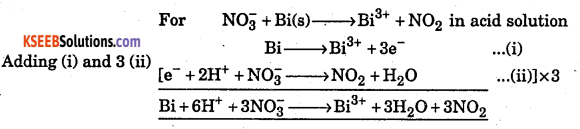
Question 33.
Cr2O72- + C2H4O– → C2H4O2 + Cr3+ in acid solution by ion electron method.
Answer:
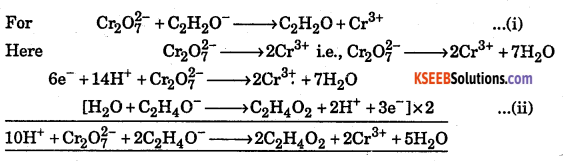
Question 34.
MnO4 + H2C2O4 → Mn2+ + CO2 in acid solution by ion electron method.
Answer:
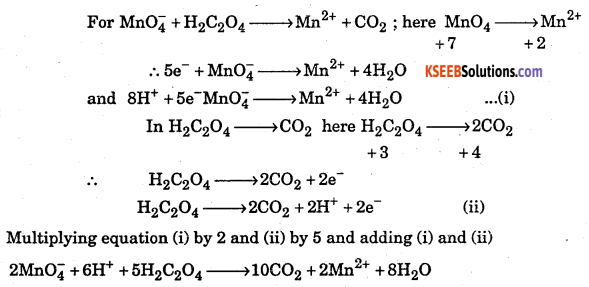
![]()
Question 35.
A cell is prepared by dipping a zinc rod in 1M ZnSO4 solution and a lead rod in 1 M Pb(NO3)2 solution. The standard electrode a potentials for Pb+2/Pb and Zn+2 /Zn electrode are -0.126 V and -0.763 V respectively. _
(a) How will you represent the cell ?
(b) Write the half cell reactions and the overall cell reaction.
Answer:
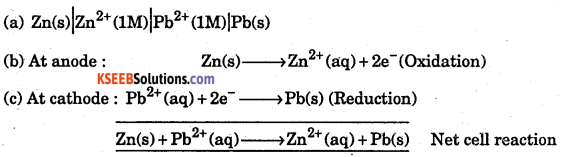
Question 36.
Copper dissolves in dilute HNO3 but not in dilute HCl. Why ? Explain.
![]()
Answer:
Electrode potential of Cu2+/Cu is higher than H+/H2 and so H+ ions cannot oxidize Cu to Cu2+ ions and therefore copper does not dissolve in dilute HCl. On the other hand, the electrode potential of NO3– ion i.e., NO3–/NO electrode is higher than that of copper electrode and hence it can oxidize Cu to Cu2+ ion; so copper dissolve in dilute HNO3.
Thus copper dissolved in dilute HNO3 due to oxidation of Cu by NO3– ions and not by H+ions.
1st PUC Chemistry Redox Reactions Five Marks Questions and Answers
Question 1.
Write the following redox reactions using half equations.
(i) Zn(s) + PbCl2 (aq) → Pb(s) + ZnCl2(aq)
(ii) 2Fe3+ (aq) + 2e– (aq) → I2(s) + 2Fe2+ (aq)
(iii) 2Na(s) + Cl2 (g) → 2NaCl(s)
(iv) Mg(s) + Cl2(s) → MgCl2(s)
(v) Zn(s) + 2H+ (aq) → Zn2+ (aq) + H2 (g)
In each of the reactions given above, mention
(i) Which reactant is oxidized ? To what ?
(ii) Which reactant is the oxidizer ?
(iii) Which reactant is reduced ? To what ?
(iv) Which reactant is the reducer ?
Answer:
(i) Zn → Zn2+ + 2e– (oxidation), Pb2+ → 2e– +Pb (reduction) Zn is oxidized to Zn2+, Pb2+ is reduced to Pb; Pb2+ is the oxidizer and Zn is the reducer.
(ii) 2Fe3+ + 2e– → 2Fe2+ (reduction), 2l– → I2 +2e– (oxidation)
Fe3+ is reduced to Fe2+, I– is oxidized to I2; I– is the reducer and Fe3+ is the oxidizer.
(iii) 3Na → 2Na+ +2e– (oxidation), Cl2 + 2e– > 2Cl– (reduction)
Na is oxidized to Na+ and Cl2 is reduced to Cl–; Na is the reducer and Cl2 is the oxidizer.
(iv) Mg → Mg2+ + 2e– (oxidation), Cl2 + 2e– → 2Cl (reduction)
Mg is oxidized to Mg while Cl2 is reduced to Cl ; Mg is the reducer and Cl2 is the oxidizer.
(v) Zn → Zn2+ +2e– (oxidation), 2H+ + 2e– >H2 (reduction)
Zn is oxidized to Zn2+ while H+ is reduced to H2;Zn is the reducer and H+ is the oxidizer.
![]()
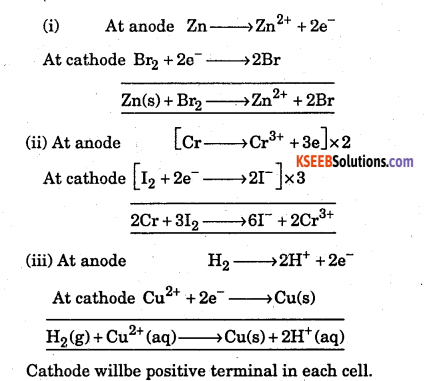
Question 2.
Write the reaction at anode and cathode and the net cell reaction in the following cells. Which electrode would be +ve terminal in each cell ?
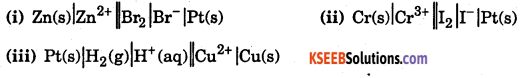
Answer:
Question 3.
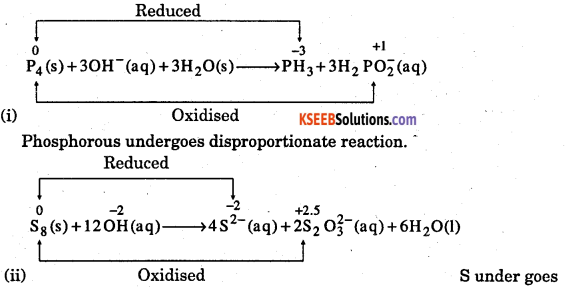
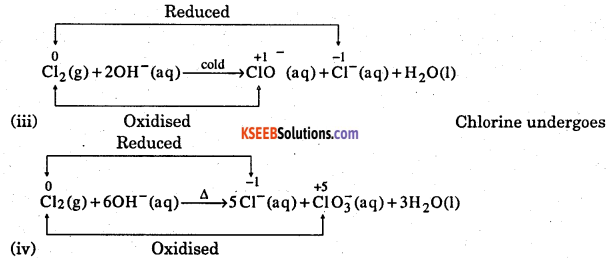
Mention which element undergoes disproportionate reaction in alkaline media.
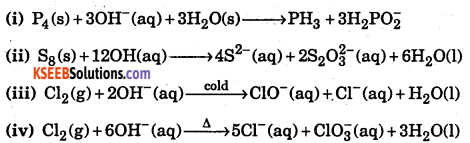
Answer:
Question 4.
Calculate emf of the following cell:
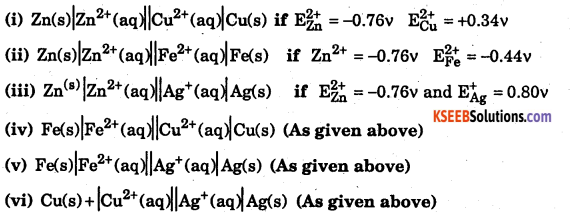
Answer:
Question 5.
In the reaction given below^ identify the species undergoing oxidation and reduction:
(i) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
(ii) CH2 = CH2(g) + H2(g) → H3C – CH3(g)
(iii) H2S(g) + O2(g) → 2S(s) + 2H2O(1)
![]()
Answer:
(i) CH4 is oxidized to CO2 while O2 is reduced to H2O.
(ii) H2S is oxidised to S while O2 is reduced to H2O.
(iii) CH2 = CH2 is reduced to CH3 – CH3 while H2 is oxidized to CH3 – CH3.
(iv) Hg2+ is reduced to Hg while O2- is oxidized to O2.
![]()
Question 6.
Using electron-transfer concept, identify the oxidation and reduction in the following redox reactions.
(a) Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
(b) 2[Fe(CN)6]4- (aq) + H2O2(aq) + 2H+(aq) → 2[Fe(CN)6]3- (aq) + 2H2O(aq)
(c) 2[Fe(CN)6]3- (aq) + 2OH–(aq) + H2O2(aq) → 2[Fe(CN)6]4-(aq) + 2H2O(l)
(d) BrO3–(aq) + F2(g) + 2OH–(aq) → BrO4–(aq) + 2F–(aq) + H2O(l)
(e) 2NaClO3(aq) + l2(aq) → 2NaIO2(aq) + Cl2(g)
Answer:
Oxidants : (a) H+ (b) H2O2, (c) [Fe(CN)6]3-, (d) Fe, (e) l2.
Reductants : (a) Zn, (b) [Fe(CN)6]4- , (c) H2O2, (d) BrO3– , (e)NaClO3
Question 7.
Why do the following reactions proceed differently ?
Pb3O4 + 8HCl → 3PbCl2 +Cl2 + 4H2O and
Pb3O4 + 4HNO3 → 2Pb(NO3)2 + PbO2 + 4H2O
Answer:
Pb3O4 is actually a stoichiometric mixture of 2 moles of PbO and one mole of PbO2, i.e., 2PbO. PbO2. In PbO2, lead is present in + 4 oxidation state, whereas in PbO, lead is present in +2 oxidation state. Since +2 oxidation state or Pb is more stable, therefore, PbO2 acts as an oxidant (oxidizing agent) and hence oxidises OF ions of HCl into Cl2. Further more, PbO is a basic oxide and hence reacts with HCl to form PbCl2 and H2O. Thus the reaction of Pb2O4 with HCl can be split into two , reactions, namely, acid-base and redox reaction as shown below :
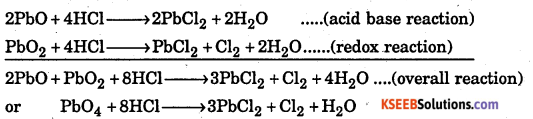
Since HNO3 is an oxidizing agent, it does not react with PbO2 which is also an oxidizing agent. Therefore, no redox reaction occurs between PbO2 and HNO3. However, acid-base reaction between PbO and HNO3 occurs as follows :
2PbO + 4HNO3 → 2Pb(NO3 )2 + 2H2O
The overall reaction of PbO3 with HNO3 can then be written as
2PbO + PbO2 + 4HNO3 → 2Pb(NO3 )2 + PbO2 + 2H2O or
Pb3O4+ 4HNO3 → 2Pb(NO3 )2 + PbO2 + 2H2O
Thus, it is the passive nature of PbO2 against HNO3that makes the reaction of Pb3O4 with HNO3 different from that with HCl.
Question 8.
Explain applications of Redox Reactions.
Answer:
1. Extraction of metals : By using a suitable reducing agent, metal oxides can be ‘ reduced to metals. For example, Fe2O3 is reduced to iron in the blast furnace using coke as the reducing agent.
Fe2O3(s) + 3C(S) → 2Fe(s) + 3CO(g)
Simialrly, Al2O3 is reduced to aluminium by cathodic reduction in an electrolytic cell. Other metals such as lithium, sodium, potassium, magnesium, calcium, etc., are also obtained commercially by electrolytic methods.
2. Electrochemical cells or batteries : Electrochemical cells or batteries based on redox reactions are widely used in our day today life to run a number of small and big gadgets and equipments. For example storage cells are used to supply all the electrical needs of our cars, trucks, buses, trains aeroplanes, etc. Similarly, electrical energy needed in the space capsule is obtained by the reaction of hydrogen and oxygen in fuel cells which are electro chemical cells using oxygen and hydrogen electrodes.
3. Photosynthesis : Green plants convert carbon dioxide and water into carbohydrates in presence of sunlight. This reaction is called Photosynthesis and is sensitized by chlorophyll.
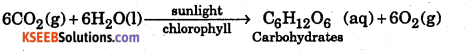
During this reaction, CO2 is reduced to carbohydrates while water is oxidized to oxygen. The energy needed for the reaction is provided by sunlight.
This reaction is a source of food for plants and animals. It also maintain a constant supply of 21% of O2 by volume in the atmosphere needed for combustion of fuels and breathing of a living creatures in the world.
4. Supply of energy : The energy required for our daily needs is obtained by oxidation of fuels. For example, oxidation of fuels such as wood, gas, kerosene, petrol, etc., produces a large amount of energy which we need for various purposes in our daily life.
Fuels (wood, petrol, kerosene, gas) + O2 → CO2 + H2O + other products + Energy
Human body also needs energy for proper functioning. This is obtained by the oxidation of glucose in our body to CO2 and water.
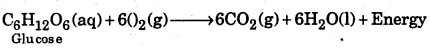
5. Production of chemicals : Many chemicals of our daily needs such as caustic soda, chlorine, fluorine, etc, are produced by electrolysis which is based on redox reaction.
6. Quantitative analysis : Redox reactions are very useful in quantitative analysis by redox titrations. These titrations involve the reactions between oxidizing and reducing agents and help in estimating the amount of unknown substances in solutions.
![]()
Question 9.
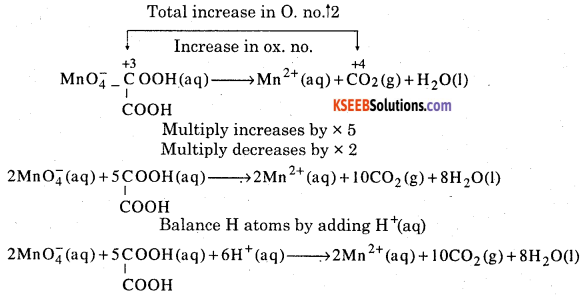
Balance the following equations in acidic medium by oxidation number
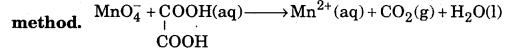
Answer:
Question 10.
Balance the following equation by ION-Electron method :
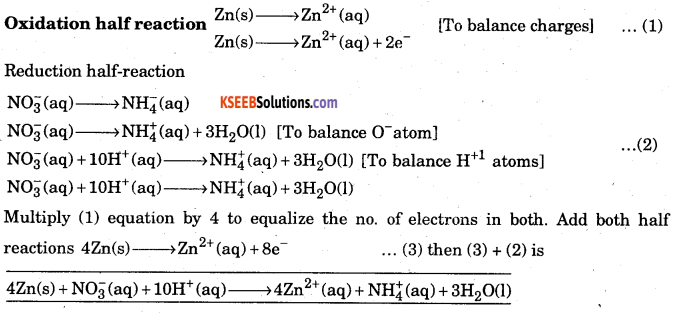
Zn(s) + NO4+ → Zn2+ (aq) + NH4+ (aq) + H2O(l)
Answer:
Question 11.
Split the following redox reaction in the oxidation and reduction half reactions:
(a) 2K(s) + Cl2(g) >2KCl(s)
(b) 2Al(s) + 3Cu2+(aq) >2Al3+(aq) + 3Cu(s)
Answer:
(a) Let us write the oxidation numbers of the atoms of all the reactants and products taking part in reaction.
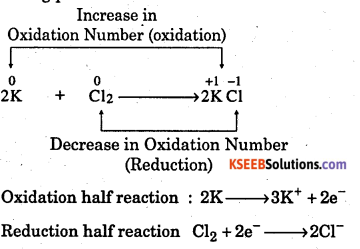
(b) Let us write the oxidation numbers of the atoms of all the reactants and products taking part in the reaction.
Increase in Oxidation Number
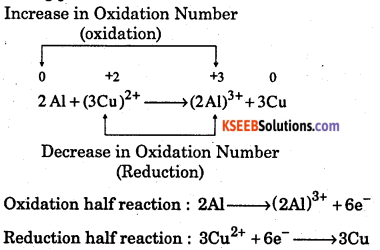
Question 12.
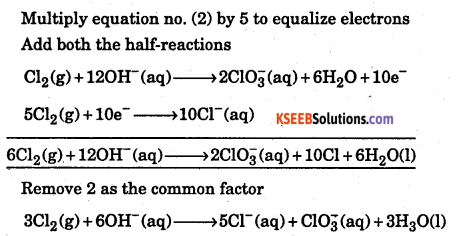
Balance the following reaction by ION-ELECTRON method Cl2(g) + OH– → Cl– (aq) + CIO3– (aq) + H2O (in basic medium)
Answer:
Cl2(g) + OH– → Cl– (aq) + CIO3– (aq) + H2O

Cl2(g) + 6H2O(l) → 2ClO3–(aq) (… Go for balancing oxygen is a abasic media odd off on RHS )
Cl2(g) + 6H2O(l) + 12OH– → 2ClO3–(aq) + 12H2O(l)
Cl2(g) + 12OH– (aq) → 2ClO3–(aq) + 6H2O(l) (now balance e-)
Cl2(g) I +12OH– (aq) → 2ClO3–(aq) + 6H2O(l) + 10e– . ..(1)
Reduction half-reaction
Cl2(g) → Cl–(aq)
Cl2(g) → 2Cl–(aq)
Cl2(g) + 3e → 2Cl–(aq) …(2)
Multiply equation no. (2) by 5 to equalize electrons
Add both the half-reactions
![]()
Question 13.
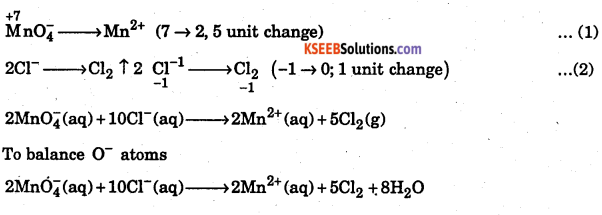
Write the over all net ionic reaction for the following change if the half reactions are correctly balanced: Cl– ion is oxidized to Cl2 by MnO2 in acidic solution: Chloride ion is oxidized to Cl2 by MnO4–(in acid solution)
Answer:
The skeletal equation is
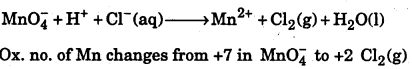
Where ox. no. of chlorine changes from -1 in Cl ion to O in Cl2
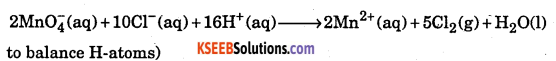
Question 12.
A cell is prepared by dipping a chromium rod in 1 M Cr2(SO4)3 solution and an ion rod in 1 M FeSO4 solution. The standard reduction potentials of chromium and iron electrodes are -0.75V and -0.45 V respectively.
(a) What will be the cell reaction ?
(b) What will be the standard EMF of the cell ?
(c) Which electrode will act as anode ?
(d) Which electrode will act as cathode ?
Answer:
The two half cell reduction equations me :
Fe2+(aq) + 2e– → Fe(s);E° =-0.45 V …(i)
Cr3+(aq) + 3e– → Cr(s);E° = -0.75V …(ii)
Since Cr3+ / Cr electrode has lower reduction potential therefore it acts as the anode while Fe2+ /Fe electrode with higher electrode potential acts as the cathode.
To equalize the number of electrons, multiply Eq. (i) by 3 and Eq. (ii) by 2. But do not multiply their E° values. Thus,
3Fe2+ (aq) + 6e– → 3Fe(s); E° = -0.45V .. .(iii)
2Cr3+(aq) + 6e– → 2Cr(s) ; E° =-0.75V …(iv)
To obtain equation for the cell reaction, subtract Eq. (iv) from Eq. (iii), we have,
2Cr(s) + 3Fe2+(aq) → 2Cr3+(aq) + 3Fe(s)
E°cell = -0.45-(-0.75V) = +0.30V Thus, the EMF of the cell = + 0.30V
Question 13.
Write the net ionic equation for the reaction of potassium dichromate (VI), K2Cr2O7 with sodium sulphate, Na2SO3, in acid solution to give chromium (III) ion and sulphate ion.
Answer:
Step-1: Write the skeleton equation for the given reaction.
![]()
Step – 2 : Find out the elements which undergo a change in oxidation number (O.N.) O.N. decreases by 3 per Cr. atom

Here, O.N. of Cr decreases from +6 in Cr2O72- to +3 in Cr3+ while that of S increases from +4 SO3-2 to +6 in SO42-
Step – 3 : Balance increase / decrease in O.N. since the total increase in O.N. is 2 and decrease is 6, therefore, multiply SO32- on L.H.S. and SO42- on R.H.S. of Eq. (ii) by 3. Combining steps 2 and 3, we have,
![]()
Step – 7 : Balance O atoms by adding H2O molecules.
![]()
Step – 7 : Balance H atoms by adding H+
![]()
Thus, Eq. (v) represents the correct balanced equation.
![]()
Question 14.
Permanganate ion react with bromide ion in basic medium to give manganese dioxide and bromate ion. Write the balance chemical equation for the reaction.
Answer:
Step – 1: Write the skeletal equation. The skeletal equation for the given reaction is :
![]()
Step – 2 :
Find out the elements which undergo a change in oxidation number (O.N.) O.N. increases by 6 per Br atom
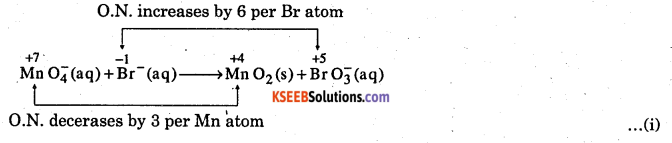
Here, O.N. of Br inceases from – 1 Br– to +5 in BrO3–, therefore, Br– acts as reductant. Further, O.N. of Mn decreases from +7 in MnO4– to +4 in MnO2, therefore, MnO4– acts as oxidant.
Step – 3 : Balance O atoms by adding H2O molecules.
![]()
Step – 4 s Balance H atoms by adding H2O and OH– ions since the reaction occurs in basic medium, therefore, add 2H2O to L.H.S. and 2OH– to R.H.S. of Eq. (iv), we have,
![]()
Question 15.
Write the half reactions for the following redox reactions.
(a) Fe2+(aq) + 2I–(aq) → 2Fe2+(aq) + I2(aq)
(b) Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
(c) Al(s) + 3Ag+(aq) → Al3+(aq) + 3Ag(s)
Answer:
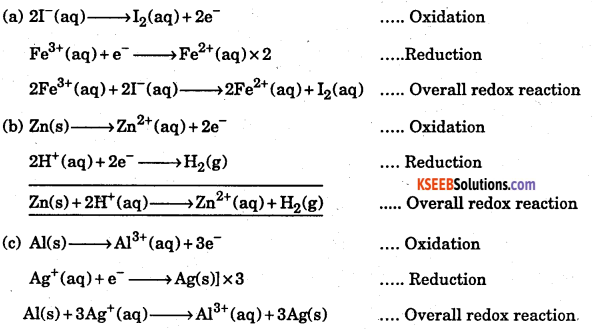
Question 16.
Balance the equation, by ion electron method.
AS2S3(S) + NO3–(aq) + H+ (aq) → AsO43-(aq) + S(s) + NO(g) + H2(l)
Answer:
Step 1 : To identify the atoms whose oxidation numbers have undergone a change. Writing the oxidation number of each atom above its symbol, we have
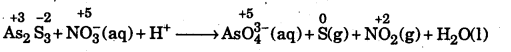
Step 2:
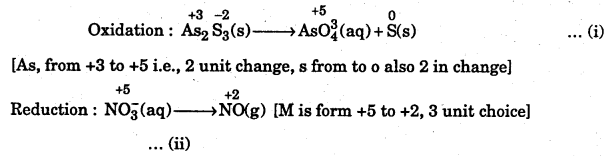
Step 3 : To balance the oxidation half Eq. (i)
(a) Balance all the atoms other than H and O. Multiply AsO43- by 2 and S by 3 on RHS of Eq. (i) we have,

(c) Balance charge by adding H+ ions. The total charge on RHS of Eq. (iv) is -16 and zero the LHS therefore, add 16H+ to RHS of Eq. (iv) we have,
![]()
(d) Balance O atoms by adding H2O molecules.
![]()
The H-atoms get automatically balanced. Thus Eq. (vi) represents the balanced oxidation half equation.
Step 4 : To balance the reduction half Eq. (ii)
(a) Balance oxidation number by adding electrons.
![]()
(b) Balance charge by adding H+ ions.
![]()
(c) Balance H+ by adding H20
![]()
Thus, Eq. (ix) represents the balanced reduction half equation Eq. (ix) by 10 and Eq,
(vi) by 3 and (ix) 10 + (vi) x 3
Step 5 :
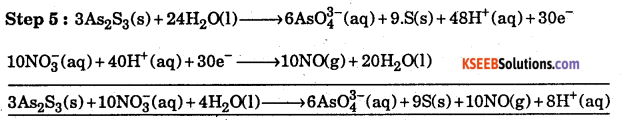
![]()
Question 17.
Permanganate (VII) ion, in basic solution oxidizes iodide ion 1- to produce molecular iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent this redox reaction.
Answer:
Step 1 s Write pie skeletal equation for the given reaction.
![]()
Step 2 : Write the O.N. of all the elements above their respective symbols.

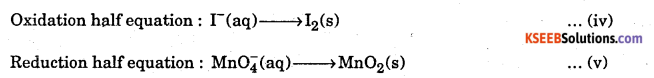
Step 4 : To balance oxidation half Eq. … (ii)
(a) Balance all atoms 2I–(aq) → I2(s) … (iv)
(b) Balance O.N. by adding electrons.
2I–(aq) → I2(s) + 2e– …(v)
Charge on either side of Eq(v) is balanced. Thus, Eq. (v) represents the balanced oxidation half equation.
Step 5 : Balance the reduction half equation (iii)
Balance O.N. by adding electrons.
![]()
Step 6: To balance the electrons lost in Eq. (v) and gained in Eq. (viii) Equation (v) x 3 + Equation (viii) x 2 we have,

This represents are final balanced redox equation.
Question 18.
In passing chlorine gas through a concentrated solution of alkali, we get chloride and chlorate ions. Obtain balanced chemical equation for this reaction by ion -electron method.
Answer:
Step 1: Write the skeletal equation for the given reaction.
![]()
Step 2 : Write the ON of all the elements above their respective symbols O.N. of Cl increases by 5 per Cl atom.
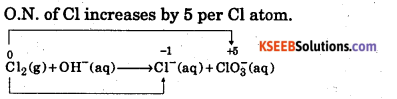
O.N. of Cl decreases by 1 per Cl atom Total increase = 2 x 5 = 10 Total decrease = 2 x -1 = -2
Step 3 : Find out the oxidant and the reductant and split the skeletal Eq (i) into two half reactions.
Step 4 : To balance the reduction half equation (ii). (a) Balance all atoms.
Cl2(aq) →2Cl–(aq) … (iv)
(b) Balance oxidation number by adding electrons.
Cl2(g) + 2e– →2Cl–(aq) … (v)
represents the balanced reduction half reactions.
Step 5 : To balance the oxidation half equation (iii)
(a) Balance all atoms Cl2(g) »2ClO3–
(b) Balance ON by adding electrons.
Cl2(g) → 2ClO3–(aq) + 10e– … (vi)
(c) Balance charge by adding OH” ions
Cl2(g) + 120H–(aq) → 2ClO3–(aq) + 10e– … (ix)
(d) Balance O atoms, the RHS of Eq. (viii) contains six O atoms but on the LHS there are 12. Therefore, add 5H2O to the RHS, we have
![]()
Eq (ix) represents the balanced oxidation half equation.
Step 6 : (v) x 5 + (ix) we have
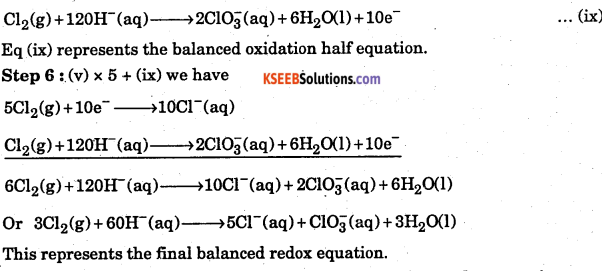
This represents the final balanced redox equation.
Question 19.
Suggest a scheme of classification of the following redox reactions.
(a) N2(g) + O2(g) →2N(Xg)
Ob) 2Pb(NO3)2(s) → 2PbO(s) + 2NO2(g) + 1/2 O2(g)
(c) NaH(s) + H2O(1)→ 4NaOH(aq) + H2(g)
(d) 2NO2(g) + 20H–(aq) → NO2–(aq) + NO3– (aq) + H2O(l)
Answer:
(a)
![]() In this reaction, the compound nitric oxide is formed by combination of elemental substances like nitrogen and oxygen. Since the oxidation number of nitrogen increases from 0 to +2 and of oxygen decreases from 0 to -2 therefore, it is a combination redox reaction.
In this reaction, the compound nitric oxide is formed by combination of elemental substances like nitrogen and oxygen. Since the oxidation number of nitrogen increases from 0 to +2 and of oxygen decreases from 0 to -2 therefore, it is a combination redox reaction.
(b)
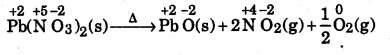
In this reaction, lead nitrate decomposes to form three products, viz, lead oxide, nitrogen dioxide and oxygen. Since the oxidation number of nitrogen decrease from +5 in lead nitrate to +4 in NO2 in O2, therefore, it is a decomposition redox reaction.
(c)
![]()
In this reaction, hydrogen of water has been displaced by hydride ion to form dihydrogen gas. Therefore, it is a displacement reaction. Since in this reaction, the oxidation number of hydrogen increases from -1 in hydride ion to zero in dihydrogen gas and that of hydrogen decreases from +1 in water to zero in dihydrogen, therefore, it is a displacement redox reaction.
(d)
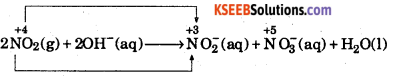
This is a disproportionation reaction since here the oxidation state of nitrogen decreases from +4 in NO2 to +3 in NO2– ion, as well as increases from +4 in NO2 to +5 in NO3 ion.
Question 20.
Calculate the oxidation number of the element underline in each of the following cases,
(a) Al in Al2O3
(b) P in P2O5
(c) S in Na2S2O3
(d) Cl in NaClO3
(e) Mn in KMnO4
(f) HA4Cl4
(g) Na2PdCl4
(h) K4MO2Cl8
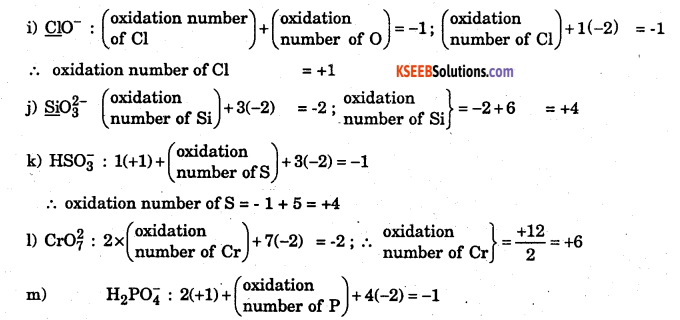
(i) ClO–
(j) Si032–
(k) H2PO4–
(1) Cr2O72–
(m) H2PO4–
Answer:
Solution : The rule used here is that the algebraic sum of the oxidation numbers of all the atoms a molecule is zero.
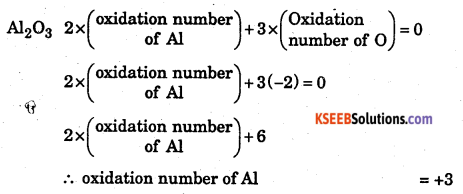
b) P2O5 : 2 x (oxidation no. of P) + 5(-2) = 0
oxidation of P = pp = +5
c) Na2S2O3 : 2(+l) + 2(oxidation no. of S) + 3(-2) = 0
2(oxidation no. of S) = 4
d) NaClO3 : 1(+1) + (oxidation no. of Cl) +3 (-2) = 0
oxidation no. of Cl = +6 – 1 = +5
e) KMnO4 : 1(+1) + (oxidation no. of Mn) + 4(-2) = 0
+1 + (oxidation no. of Mn) – 8 =0
∴ oxidation no. of Mn = + x7
Answer:
Solution : The rule used here is that the algebraic sum of the oxidation numbers of all the atoms in an ion is equal to the net charge on the ion.
f) HA4Cl4 1(ON of H) + 1C (ON of A4) + (ON of Cl) = 0
(+1) + x + 4(-l) = 0 => l + x-4 = 0 => x = +3
g) Na2PdCl2 = 2(+l) + x + 4(-l) = 0 => 2+x-5=0 => x = +2
h) x be ON to be find
K4MO2Cl8 = 4(+l) + (x)2 + 8(-l) = 0
4 + 2x – 8 = 0 => 2x – 4 = 0 => x = +2
![]()
Question 21.
What is oxidation number of sulphur in the following molecules / ions (n) sinH2S sin SO42- sinH2SO4
Answer:
(i) The oxidation number of Hydrogen is +1, and s = x than

(ii) SO42- let x be ON of s ; Oxidation Number of oxygen is -2

(iii) H2SO4 let x, +1, -2 be on of S, H and O
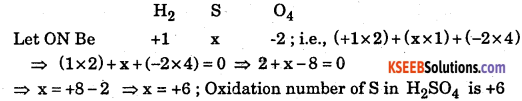
Question 22.
Find the oxidation number of the underlined atoms.
(a) CCl4
(b) CH4
(C) ClO3–
(d) BrF3
(e) Na2 B4O7
(f) HClO3
(g) HPO2 –
(h) Fe3 O4
Answer:
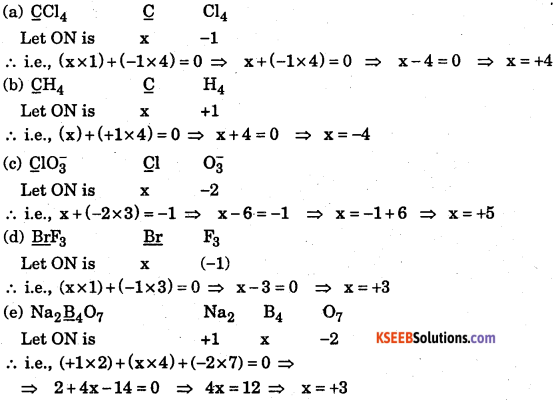
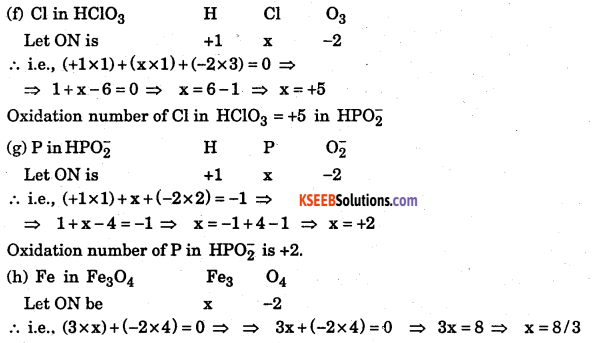
![]()